 |  |
Intravitreal injections of anti-VEGF are FDA-approved for a number of ocular conditions and have been shown to decrease macular edema and improve vision.1-5 The most common are aflibercept (Eylea), ranibizumab (Lucentis) and bevacizumab (Avastin).
Aflibercept acts as a decoy receptor that binds to and inhibits the activation of VEGF receptors on endothelial cells. It can treat wet AMD, macular edema from vein occlusion, diabetic macular edema and diabetic retinopathy. Ranibizumab binds to active VEGF and prevents interaction with receptors on endothelial cells. The drug treats the same conditions as aflibercept and is also indicated for intravitreal use in myopic choroidal neovascularization. While bevacizumab’s use is considered off-label, the drug has achieved similar success rates as aflibercept and ranibizumab.6
The Process
Though optometrists cannot perform these injections, it’s helpful to understand it for comanagement purposes.
On the day of the procedure, the patient’s visual acuities and intraocular pressures will be measured and the eye receiving treatment will be noted by marking the forehead. The patient will put on a hair net and be put in the supine position. A lid speculum will be inserted to prevent blinking. The patient will be instructed to look up and away and be still.
The injection site is inferotemporal to the limbus due to its ease of accessibility. Using a caliper, the retina specialist administering the injection will measure the distance between the two; it should be 4.0mm in phakic eyes to prevent inadvertent puncture of the natural lens and 3.5mm in aphakic and pseudophakic eyes. The injection site will be prepared using topical proparacaine 0.5%, povidone-iodine 5.0% and lidocaine hydrochloride 3.5%; povidone-iodine swab sticks will be used to sterilize the eyelids and surrounding tissue.
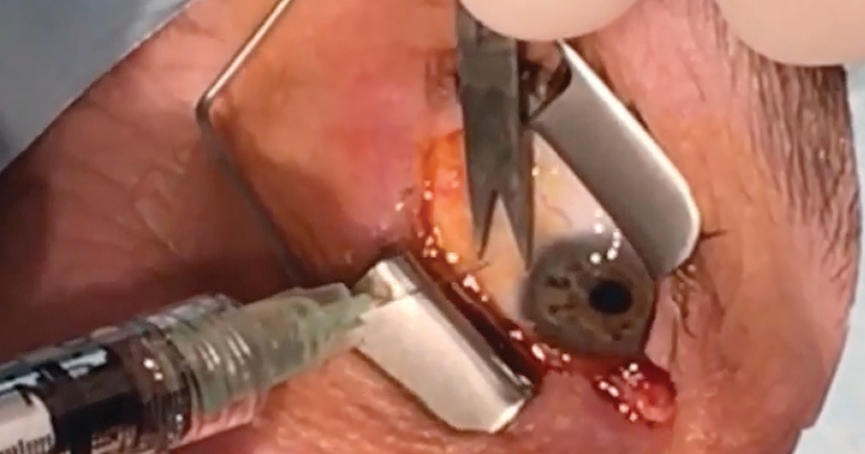 |
| A patient undergoes an anti-VEGF injection. Click image to enlarge. |
The patient should not speak, cough or sneeze, as streptococcal isolates in saliva are three times more likely to cause endophthalmitis post-intravitreal injection than post-intraocular surgery.7 Everyone in the room should be wearing a mask.
One drop of povidone-iodine 5.0% will be instilled directly onto the injection site, then the surgeon will insert the needle perpendicular to the sclera and through the underlying pars plana, and inject the medication into the vitreous chamber. The patient’s gross visual acuity will be checked to ensure the increase in vitreous chamber volume did not cause a significant decrease in retinal profusion. The patient should be able to accurately count fingers within seconds of the injection. After the speculum is removed, the eyelid and eye will be rinsed with saline to remove any residual povidone-iodine to prevent superficial punctate keratitis.
Intravitreal injections may cause a patient to see a black spot due to an air bubble in the medication. This is not serious, and the air bubble should reabsorb in about a week. A subconjunctival hemorrhage may occur but should also resolve spontaneously and without complication in a week. Significant side effects following intravitreal injections are rare.8 The patient should avoid dusty environments and eye-rubbing and return to the doctor if they experience decreased vision, pain and redness or flashes and floaters.
Mr. Bauer and Ms. Tran are fourth-year optometry students at Pacific University in Forest Grove, OR.
Dr. Skorin is a consultant in the Department of Surgery, Community Division of Ophthalmology in the Mayo Clinic Health System in Albert Lea, MN.
| 1. Talks J, Daien V, Finger RP, et al. The use of real-world evidence for evaluating anti–vascular endothelial growth factor treatment of neovascular age-related macular degeneration. Surv Ophthalmol. 2019;64(5):707-19. 2. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina .2006;26(8):859-70. 3. Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology .2013;120(11):2292-9. 4. Anti-VEGF treatment for prevention of PDR/DME. NIH Clinical Trials. clinicaltrials.gov/ct2/show/NCT02634333. September 27, 2018. Accessed December 19, 2019. 5. Kress B. Anti-VEGF: Where are we now? www.reviewofoptometry.com/article/antivegf-where-are-we-now. March 15, 2019. Accessed December 19, 2019. 6. Dashti-Khavidaki S, Abdollahi M. Intravitreal administration of bevacizumab: pros and cons. Daru J Pharm Sci. 2015;23(1):27. 7. McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina. 2011;31(4):654-61. 8. Sachdeva MM, Moshiri A, Leder HA, et al. Endophthalmitis following intravitreal injection of anti-VEGF agents: long-term outcomes and the identification of unusual micro-organisms. J Ophthalmic Inflamm Infect. 2016;6(1):2. |

