The Tear Film and Ocular Surface Society (TFOS) delivered a landmark manuscript when it issued its second Dry Eye Workshop (DEWS II) report in 2017. That research highlighted the relationship between the intricately connected structures of the ocular surface.
The take-home point is that there rarely is just one structure affected when tear homeostasis is disrupted. Dry eye states, for instance, worsen allergic reactions, and those allergic reactions cause more inflammation, which in turn, worsens ocular dryness. Inflammation is both the cause and effect of dry eye states. It’s a vicious cycle.
TFOS’s original DEWS report, published in 2007, defined the lacrimal functional unit (LFU) as an integrated system comprised of the lacrimal glands, the ocular surface, the eyelids and the sensory/motor nerves that connect them all.1
This article reviews the key areas of the LFU and how they are altered when ocular allergy and dry eye emerge.
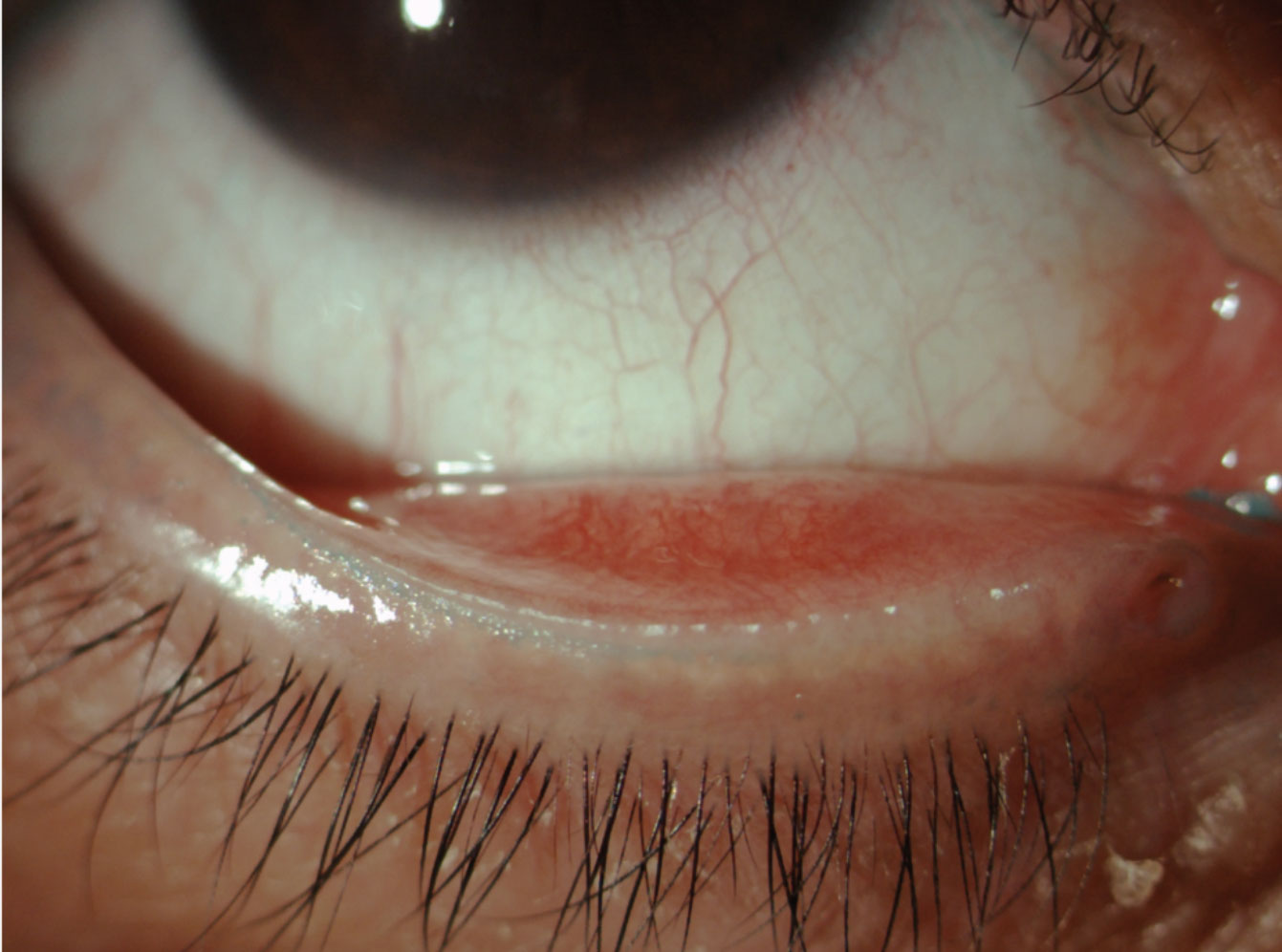 |
| Fig. 1. This patient’s line of Marx is visible thanks to vital dye staining. Click image to enlarge. |
The Value of Healthy Lids
In general, when patients experience problems with their eyelids, they are often gradual and likely developed before obvious symptoms were noticed. The lids, and subsequently the palpebral conjunctiva, are two areas that optometrists must investigate extensively.
The eyelids contain three glands—Zeis, Moll, meibomian—that come together to create the lipid layer, comprised of low polarity (wax and cholesterol esters) and high polarity lipids (triglycerides, free fatty acids and phospholipids). The main function of these lipids is to create a uniform, protective top layer of the tear film.2 While small—only 3uL thick—the tear film creates lubrication, as well as antimicrobial protection, nutrition, maintenance of corneal transparency and surface stem cell population. It’s vital for the removal of debris and the preservation of the quality of the image projected to the retina.2-4
Blinking occurs approximately once every three to four seconds in most patients. However, research shows digital devices are reducing blink rates to 4.5 per minute.5
This is one lifestyle factor that can cause the LFU to dysfunction, setting off the vicious dry eye cycle. A normal functioning eye would flush away inflammatory mediators that suppress T-cell activation and inhibit complement-mediated tissue damage by blinking and tear drainage from the ocular surface. With fewer blinks, the patient may be at risk for meibomian gland atrophy and ultimately chronic ocular surface disease.6
LFU Examination
Evaluating the external eyelid begins during initial observation. This exam requires optometrists to observe lid positioning, blink rates and lid closure before putting their patient in front of a slit lamp. These evaluations are especially important in geriatric patients, as their orbicularis muscle may lose tension and lagophthalmos and lid congruity conditions may be present. Observing the eyelid margins may lead to additional causes for a patient’s symptomatology.
Lid wiper epitheliopathy can be a contributing factor to many debilitating symptoms.7 A patient who presents with a normal appearing blink rate and function but is symptomatic of dry eye or contact lens discomfort warrants an evaluation of this structure. The lid wiper is the anatomical area of the palpebral marginal conjunctiva in the upper and lower lids that is in contact with the globe. In the upper lid, it extends from the crest of the sharp posterior (inner) lid border (the mucocutaneous junction) to the subtarsal fold superiorly, and from the medial upper punctum to the lateral canthus horizontally in the lower lid.7 Lid eversion is a simple, useful diagnostic assessment for any patient presenting with symptoms of dry eye or contact lens discomfort.
Lid wiper epitheliopathy is also associated with decreased tear film stability, contact lens wear and lid-parallel conjunctival folds.8 It’s best observed with vital dye staining—in particular, lissamine green, which can unveil significant dryness secondary to frictional and mechanical forces (such as contact lens wear).9
The mucocutaneous junction, also known as the line of Marx (LOM), runs parallel to and away from the orifices of the meibomian glands along the conjunctival border in normal patients and becomes anteriorly displaced to the orifices with increasing irregularity.10 Researchers still aren’t sure whether meibomian gland dysfunction (MGD) is caused by this anterior displacement of the LOM or if MGD precedes its displacement.
It is possible to view the superior LOM without lid eversion but research shows lissamine green is the best way to observe keratinized debris (Figure 1).11
Debridement of LOM is a clinically significant method for improving symptoms.12 It also provides meibomian gland production in some severe dry eye patients.12 The mechanical debridement of the LOM and the lid margin removes keratin from the meibomian gland orifices that can obstruct lipid expression to the ocular surface.13 This technique can provide its own statistically significant symptom relief and improve meibomian gland function.14
Carefully evaluating the base of the lashes on slit lamp examination can help expose an abundance of Demodex folliculorum or Demodex brevis.15 Run amock, these mites can both create eyelid inflammation (blepharitis) and inflame rosacea that, in turn, affect the meibomian glands’ ability to secrete meibum—another vicious cycle.16,17
Research presented at ARVO in 2017 demonstrated that cylindrical dandruff was pathognomonic for the presence of Demodex (Figure 2).17,18 Additionally, patients with more lid laxity also had higher incidence of Demodex.17,18 Treatments include tea tree oil and examination for clinical sign of these mites should be done during a general examination.
Many chemicals used in cosmetics affects the ocular surface. In fact, oil-based makeup can provide sustenance for Demodex, so ODs should advise patients regarding daily cosmetic hygiene practices and consistent removal of cosmetics. Those with a Demodex diagnosis should avoid oil-based make-up products. Even those without Demodex-related issues who do not remove make-up products report more complaints of dry eyes than those who do remove those products.20
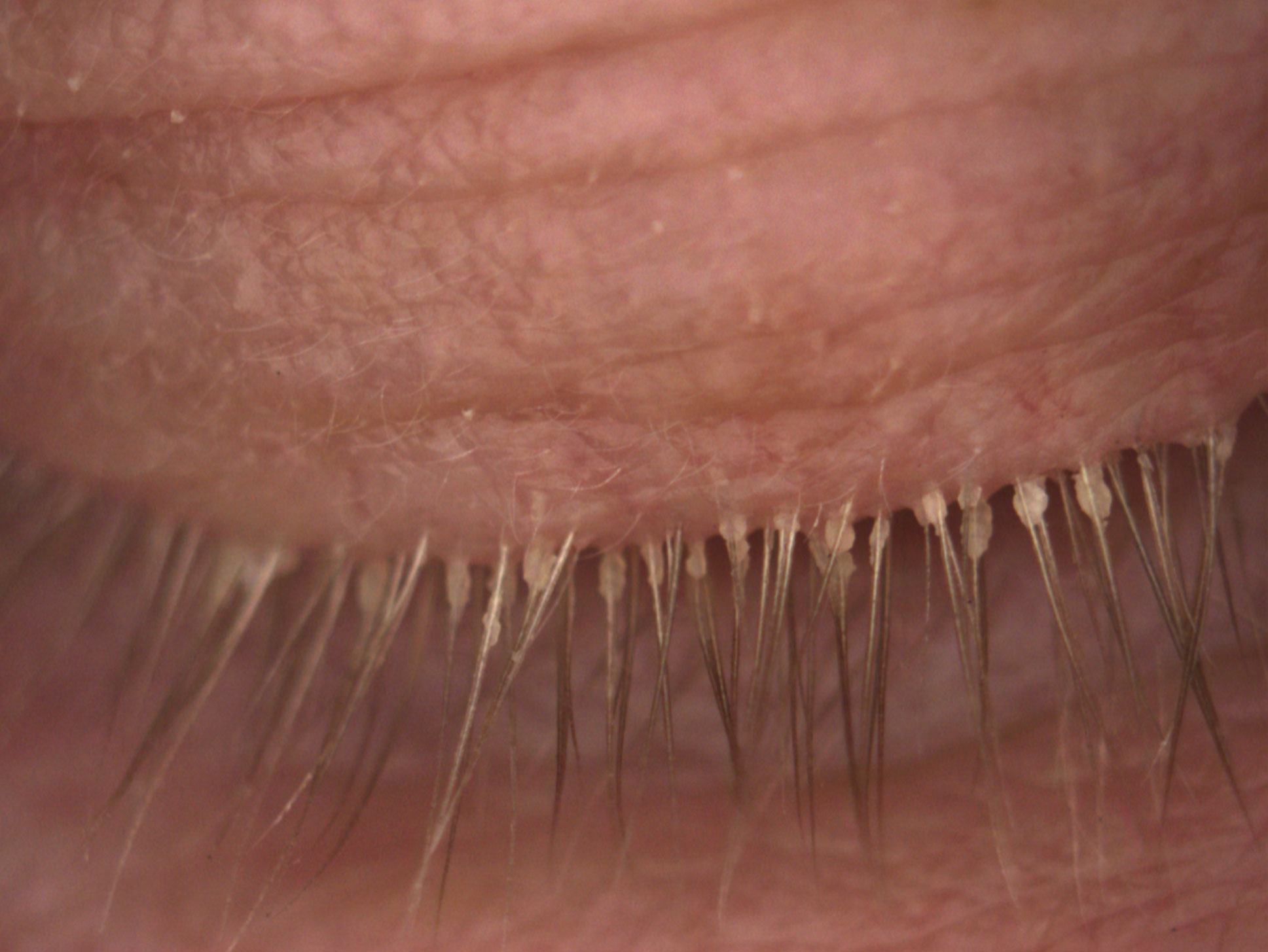 |
| Fig. 2. This patient’s lashes show cylindrical dandruff secondary to the presence of Demodex. Click image to enlarge. |
Meibomian Gland Dysfunction
Research suggests MGD is the most frequent cause of DED.21 Diagnosing MGD requires a combination of collecting patient symptoms—such as burning, itching, irritation, foreign body sensation and dryness —and diagnostic imaging—such as meibography, tear break-up time, the Korb Meibomian Gland Evaluator (Johnson & Johnson Vision) and Schirmer testing (Figure 3).22
MGD and DED are not mutually exclusive. MGD may result, as discussed, from eyelid inflammation, but it can also arise from conjunctival inflammation, corneal damage, tear film instability, microbiological changes associated with DED or any combination of those.21 Gland dropout, blockage and inflammation can all cause stasis. Over time, this leads to chronic proliferation of bacteria and can provide an environment Demodex, ultimately increasing lid and conjunctival inflammation, thus perpetuating the chronic inflammatory cycle of DED.21,23,24
Allergy
Ocular allergy can significantly alter the DED cycle by initiating an inflammatory response in the ocular surface, which in turn leads to tear film instability.25 The DEWS II includes allergic conjunctivitis among DED’s likeliest risk factors.25
Ocular allergy is typically classified into two main categories: common keratoconjunctivitis (seasonal and perennial) and rarer keratoconjunctivitis (vernal and atopic). Seasonal and perennial conjunctivitis are immunoglobulin E (IgE)–mediated hypersensitivity responses, typically presenting with mild-to-moderate signs and symptoms of ocular allergy. Allergic conjunctivitis can be distinguished from Demodex based on the location of the itch. In allergy, it is directed toward the conjunctiva, whereas Demodex itching is experienced along the lid.
Vernal and atopic keratoconjunctivitis are T-helper–mediated responses, often presenting with more complex, severe chronic inflammatory responses.
Several studies show an association between ocular allergy and reduced tear break-up-time (TBUT).25,28-31 A 2017 study demonstrates how patients with ocular allergy develop morphological changes to their meibomian glands, which could be attributed to either ocular inflammation or damage from scratching and eye-rubbing, or both.32
The DEWS II research includes tear osmolarity in its diagnostic protocol, as it is a core mechanism of dry eye disease.23 One study looked at osmolarity in patients with allergic rhinoconjunctivitis, finding values of 318mOsm/l to 324mOsm/l.34-36 Another study found increased levels of inflammatory markers matrix metalloproteinase-1, -2 and -9 in patients with vernal keratoconjunctivitis, which is significant, as increased levels of MMP-9 can lead to increased ocular surface staining and increased symptoms of dry eye disease.34-36
Neurosensory abnormalities also play a key role in ocular surface disease.37 Hyperosmolarity of the tear film and ocular surface inflammation—both of which can result from allergic reactions—can change corneal sensory receptors by inducing peripheral sensitization and even nerve damage. Symptoms of ocular allergy and DED frequently overlap and are mediated by corneal sensory innervation. DED and ocular allergy are not mutually exclusive, and ODs must take care to examine the entire ocular surface to ensure patients receive appropriate integrated management.
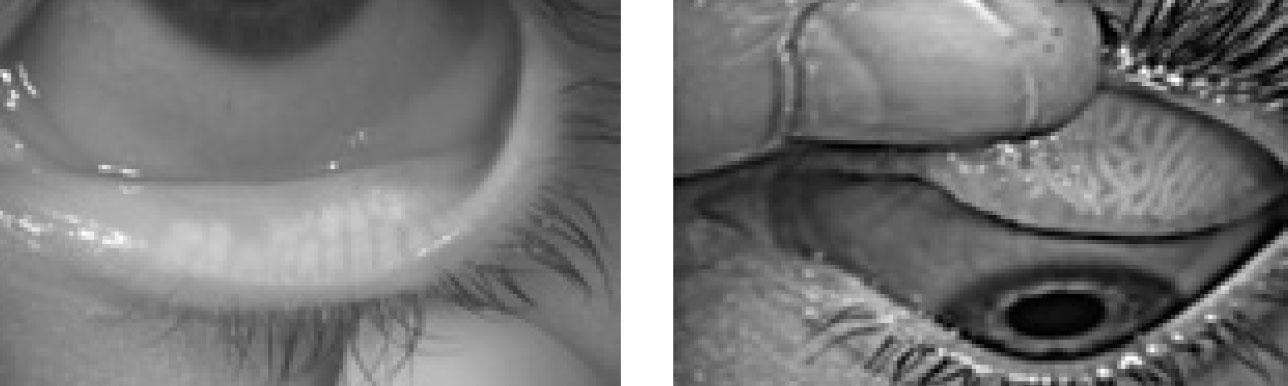 |
| Fig. 3. These meibography images of an upper and lower lid demonstrate atrophy, truncation and tortuosity of the meibomian glands. Click image to enlarge. |
Right on Target
The symptomology of a patient’s dry eye does not always match their clinical signs. This makes clinical management difficult. However, as we begin to understand the disease further, this discrepancy is becoming less of an issue. Many practitioners have incorporated protocols to help develop more specific diagnoses and more targeted management of DED.
Diagnostic dyes such as sodium fluorescein, lissamine green dye and rose bengal are among the most common ways to observe signs of dry eye damage. In 2016, investiagtors noted that clinical dyes used as a means to determine the absence of tear abnormalities could produce a false negative in many cases ruled as ‘symptoms not matching clinical signs’.37 The research argued that a single instillation of a dye may not be sufficient to elicit evidence of ocular surface or lid wiper epitheliopathy associated with desiccation.37 A second instillation may possibly show clinical evidence.37 The concentration and volume of the dye per manufactured strip could also play a factor in results.37
Many practitioners have adopted point-of-care testing to assist with DED diagnosis and monitoring. Hyperosmolarity has been described as a primary marker of tear film integrity and is often higher in patients diagnosed with DED.38 Measurement of tear osmolarity has a high-positive predictive value and should not be used as a standalone test. An unstable tear film will cause osmolarity readings to fluctuate, indicating the patient may need a change in management. MMP-9 immunoassay measurement provides information regarding the presence of inflammation on the ocular surface. Inflammation may be present before the clinical signs of dry eye, contributing to increased corneal desquamation and corneal surface irregularity. Point-of-care testing assists with the management of the patient, helping the practitioner quantify and monitor the patients response to therapies.
Meibomian gland evaluation through expression and meibography has become an essential part of the clinical examination. However, a common concern with meibography is how to appropriately use the information gathered to help classify a patient’s level of MGD. Heiko Pult’s grading scale was determined to be the most agreeable between grading clinicians and has been used to grade meibomian gland atrophy (MGA) in multiple research papers and posters.30
Lifestyle
Environment and geographic location may contribute factors to ocular surface disease. Temperature, humidity and pollen counts can affect the signs and symptoms of dry eye. Expectedly, as humidity levels increased, symptomology decreased. However, in times of higher temperature without humidity symptoms of dry eye increased. In addition, as pollen counts elevated, symptoms increased, more lower lid staining was recorded and non-invasive tear break up time decreased.40,41 The use of digital devices is growing and with increased use, eye care practitioners have noted higher prevalence of dry eye signs and symptoms among all age groups, including children. The potential adverse effect of this increased digital use may interfere with work and school performance, productivity and quality of life.
A study presented at ARVO in 2016 demonstrated that, regardless of age, patients are using an increased number of digital devices for multiple hours throughout the day contributing to dry eye symptoms and vision fluctuation.42
The ocular surface is complex and complaints of ocular discomfort are often not exclusive to one disease process. Advancements in anterior diagnostic testing have furthered our understanding of how changes to the structures of the anterior eye contribute to ocular surface disease processes. Optometrists are in a unique position to make a true difference in our patient’s lives and there is much every practitioner can do to make a positive difference to the health our patient’s eye. By reminding ourselves how eyelid morphology, allergic processes and dry eye progression are connected, careful examination of every patient will lead to more preventative and customized management.
Dr Halleran is a private practice owner in Clarenville Newfoundland Canada and serves as an adjunct clinical instructor for Waterloo School of Optometry.
Dr. Harthan is a professor at the Illinois College of Optometry and chief of the Cornea Center for Clinical Excellence at the Illinois Eye Institute.
1. International Dry Eye WorkShop. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul Surf. 2007;5(2):75-92. 2. Green-Church K, Butovich I, Wilcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Invest Ophthalmol Vis Sci. 2011;52(4):1979-93. 3. Sridhar M. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66(2):190-4. 4. Gulati S, Jain S. Ocular pharmacology of tear film, dry eye and allergic conjunctivitis. Handb Exp Pharmacol. 2017;242:97-118. 5. Bentivoglio A, Bressman S, Cassetta E, et al. Analysis of blink rate patterns in normal subjects. Mov Disord. 1997;12(6):1028-34. 6. Schaumberg D, Sullivan D, Buring J, Dana M. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318-26. 7. Korb D, Greiner J, Herman J, et al. Lid-wiper epitheliopathy and dry-eye symptoms in contact lens wearers. CLAO J. 2002;28(4):211-6. 8. Li W, Yeh T, Leung T, et al. The relationship of lid wiper epitheliopathy to ocular surface signs and symptoms. 2018;59(5):1878-87. 9. Efron N, Brennan N, Morgan P, Wilson T. Lid wiper epitheliopathy. Prog Retin Eye Res. 2016;53(7):140-74. 10. Jester J, Parfitt G, Brown D, et al. Meibomian gland dysfunction: hyperkeratinization or atrophy? BMC Ophthalmol. 2015;15 Suppl 1:156. 11. Korb D, Blackie C. Marx’s line of the upper lid is visible in upgaze without lid eversion. Eye Contact Lens. 2010;36(3):149-51. 12. Ngo W, Srinivasan S, Schulze M, Jones L, et al. Repeatability of grading meibomian gland dropout using two infrared systems. Optom Vis Sci. 2014;91(6):658-67. 13. Zarei-Ghanavatia S, Heravian Shandiz J, Abrishami M, Karimpour M. Comparison of mechanical debridement and trans-epithelial myopic photorefractive keratectomy: A contralateral eye study. 2019;31(2):135-41. 14. Korb DR, Blackie CA. Debridement-scaling: A new procedure that increases meibomian gland function and reduces dry eye symptoms. Cornea. 2013;32(12):1554-7. 15. Jarmuda S, O’Reilly N, Zaba R, et al. Potential role of Demodex mites and bacteria in the induction of rosacea. J Med Microbiol. 2012;61:1504-10. 16. Fromstein S, Harthan J, Patel J, Opitz D. Demodex blepharitis: clinical perspectives. Clin Optom (Auckl). 2018;10:57-63. 17. Hom M, Mastrota K, Schachter S. Demodex. Optom Vis Sci. 2013;90(7):e198-205. 18. Halleran C, O’Dell L, Harthan J et al. An evaluation of the presence of Demodex and its influence on meibomian gland structure. Invest Ophthalmol Vis Sci. 2017;58:ARVO. 19. Rather P, Hassan I. Human Demodex Mite: The Versatile Mite of Dermatological Importance. Indian J Dermatol. 2014;59(1):60–6. 20. O’Dell L, Periman L, Sullivan A, et al. An evaluation of cosmetic wear habits correlated to ocular surface disease symptoms. Invest Ophthalmol Vis Sci. 2017;58:ARVO E-Abstract:495-A0420. 21. Baudouin C, Messner E, Aragona P, et al. Revisiting the vicious circle of dry eye disease: a focus on the pathophysiology of meibomian gland dysfunction. Br J Ophthalmol. 2016;100(3):300-6. 22. What is meibomian gland dysfunction? WebMD. www.webmd.com/eye-health/meibomian-gland-dysfunction#2-6. Accessed October 1, 2019. 23. Kosik-Bogacka D, Lanocha N, Lanocha A, et al., Role of Demodex folliculorum in the pathogenesis of blepharitis. Acta Ophthalmol. 2012;90(7):e579. 24. Murillo N, Aubert J, Raoult D. Microbiota of Demodex mites from rosacea patients and controls. Microb Pathog. 2014;71-72:37-40. 25. Villani E, Mantelli F, Nucci P. In-vivo confocal microscopy of the ocular surface: ocular allergy and dry eye. Curr Opin Allergy Clin Immunol. 2013;13(5):569-76. 26. Leonardi A, Motterle L, Bortolotti M. Allergy and the eye. Clin Exp Immunol. 2008;153(Suppl 1):17–21. 27. Kabat A, Sowka J. If it itches, it’s allergy...right? Rev Optom. 2011;147(6):107-8. 28. Villani E, Strologo M, Pichi F, et al., Dry eye in vernal keratoconjunctivitis: A cross-sectional comparative study. Medicine (Baltimore). 2015;94(42):e1648. 29. Chen L, Pi L, Fang J, et al., High incidence of dry eye in young children with allergic conjunctivitis in Southwest China. Acta Ophthalmol. 2016;94(8):e727-30. 30. Akil H, Celik F, Ulas F, Kara IS. Dry eye syndrome and allergic conjunctivitis in the pediatric population. Middle East Afr J Ophthalmol. 2015;22(4):467-71. 31. Kim T, Moon N. Clinical correlations of dry eye syndrome and allergic conjunctivitis in Korean children. J Pediatr Ophthalmol Strabismus. 2013;50(2):124-7. 32. Mizoguchi S, Iwanishi H, Arita R, et al. Ocular surface inflammation impairs structure and function of meibomian gland. Exp Eye Res. 2017;163:78-84. 33. Wolffsohn J, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-74. 34. Leonardi A, Brun P, Abatangelo G, et al., Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44(7):3052-8. 35. Kumagai N, Yamamoto K, Fukuda K, et al., Active matrix metalloproteinases in the tear fluid of individuals with vernal keratoconjunctivitis. J Allergy Clin Immunol. 2002;110(3):489-91. 36. Villani E, Rabbiolo G, Nucci P. Ocular allergy as a risk factor for dry eye in adults and children. Curr Opin Allergy Clin Immunol. 2018;18(5):398-403. 37. McMonnies C. The potential role of neuropathic mechanisms in dry eye syndromes. J Optom. 2017;10(1):5-13. 38. Messmer E, Bulgen M, Kampik A. Hyperosmolarity of the tear film in dry eye syndrome. Dev Ophthalmol. 2010;45:129-138. 39. Halleran C, Kwan J, Hom M, Harthan J. Agreement in reading centre grading of meibomian gland tortuosity and tortuosity. Poster presented at the American Academy of Optometry. November 2016;Anaheim CA. 40. Harthan J, Hom M, O’Dell L, Halleran C. Impact of humidity levels, temperature, breathing and heat indices on dry eye symptom scores. Poster presented at the American Academy of Optometry. October 2017;Chicago, IL. 41. Halleran C, Kwan J, Hipolito K, Harthan J, Hom MM. The role of pollen counts on the Signs and symptoms of ocular surface disease. Poster presented at the American Academy of Optometry. October 2017;Chicago, IL. 42. Schachter A, Schachter S, Kwan J, et al. Impact of digital device use on dry eye symptoms. Poster presented at the American Academy of Optometry. November 2016;Anaheim CA. |

