Annual Innovations in Eye Care IssueFollow the links below to read other articles from our annual Innovations in Eye Care issue: |
Anti-vascular endothelial growth factor (VEGF) therapy has dramatically revolutionized the management of age-related macular degeneration (AMD), diabetic macular edema and retinal vein occlusions. Many trials have shown that, worldwide, the treatment has significantly changed outcomes in patients with retinal vascular disease and reduced the incidence of blindness from these conditions.1-5 In each of these indications, the best outcomes have been shown with fixed dosing schedules.6 However, such a regimen risks either over- or undertreatment if the chosen intervals between treatments are too short or too long.7
Numerous studies have shown the challenges of bringing the efficacy demonstrated in clinical trials into the real world.8 Fixed monthly dosing is associated with huge burdens for the patient, the physician, the clinic and the health system. Patients experience inconvenience and infection risk each time an injection takes place. As most are elderly, they typically require the presence of a caregiver at office visits, extending the disruption to family members. Retina practices must maintain high inventories of medications and establish a tight workflow that allows high-volume dispensing. These activities consume precious physician and staff time that causes a backlog of cases to develop, delaying care for other patients. And the health case system as a whole must then process and reimburse it all.
Therefore, clinicians in everyday practice have turned to regimens such as PRN or treat-and-extend schedules.7-9 Outcomes obtained with PRN depend largely on the retreatment criteria employed by the treating physician and how closely these are adhered to. However, these regimens require multiple examinations, are dependent on patient compliance and do not achieve the same results as monthly treatment. The burden of treatment combined with the lack of efficacy in reactive modes of therapy creates a need for better delivery systems.
As a result, other modes of drug delivery with potentially less intervention and less need for follow up have been explored. Technologies that could extend the duration between treatments could offer a significant benefit to patients by reducing the burden of treatment while improving vision.
 |
| After inserting Roche’s investigational Port Delivery System, the surgeon pulls the conjunctiva back over the implant—leaving the device slightly visible to the naked eye. Click image to enlarge. |
Refillable Drug Reservoir
The Port Delivery System (PDS), under development by Swiss drugmaker Roche, is a durable intravitreal reservoir implant placed through a scleral incision in the pars plana in a one-time surgical procedure.
The device, roughly the size of a grain of rice, is loaded with ranibizumab (Lucentis, Genentech) via a custom needle and then inserted using standard vitreoretinal surgical techniques. After the physician makes a small pars plana incision and inserts the device, the conjunctiva is then pulled back over the implant and secured in place, similar to typical 20-gauge vitreoretinal surgery. The procedure takes a few minutes in the operating room, followed by a short recovery period.
The implant holds a small volume of a highly concentrated drug that is slowly released over a four- to six-month period, or perhaps longer. The device can then be refilled in the ophthalmologist’s office with a custom needle that is part of the drug/device combination system.
The technology was originally developed by ForSight Vision4 to improve delivery of a variety of therapeutic payloads, including small molecules that typically stay in the eye for a short time. ForSight Vision4 is the fourth start-up company to arise through ForSight Labs, which was also responsible for developing the CyPass glaucoma stent (acquired by Alcon) and a drug-eluting periocular ring for glaucoma drug delivery (acquired by Allergan).
 |
| The PDS uses this intravitreal reservoir implant to release an anti-VEGF agent over four to six months. |
ForSight Vision4 began collaborating with Roche’s Genentech unit in 2010. In late 2016, Roche acquired ForSight Vision4 and the PDS. The acquisition has also provided researchers the opportunity to apply the PDS technology to other molecules in the company’s pipeline.
A Phase I trial involving 20 patients found that the implant was well tolerated and improved best-corrected visual acuity in a manner comparable to monthly ranibizumab injections.10 On average, patients in this trial needed fewer than five refills over the course of a year, establishing proof of concept for the system.
Following the promising Phase I results, Genentech received FDA fast-track designation for the implant and initiated a Phase II clinical trial of the implant in patients with wet AMD. The Long Acting Delivery of Ranibizumab (LADDER) study is evaluating the safety and efficacy of the implant in patients who have previously responded to ranibizumab therapy, in hopes of reducing the number of office visits and injections while still achieving the maximum benefit possible from therapy. The multicenter, randomized controlled trial is evaluating the implant with three different ranibizumab concentrations vs. conventional delivery of ranibizumab 0.5mg.11
The primary study endpoint of LADDER is the time point at which a patient first requires the RPDS implant to be refilled according to protocol-defined refill criteria. The goal is to determine the pharmacodynamic relationship and the refill interval, thus defining the duration of the therapeutic effect between refills. The desired target for the ranibizumab port delivery system is four to six months between refills. The study also seeks to determine whether the efficacy is the same if the drug is delivered at a steady rate over long periods of time compared to the peaks and valleys of intermittent dosing. Enrollment has been completed and top-line data are expected later this year.
Using the Port Delivery System to administer ranibizumab (and potentially other molecules) in sustained-release fashion creates the potential for fewer office visits, fewer total interventions and comparable outcomes with much less burden. The technology, however, requires a scleral incision, one that should be performed for now in the operating room setting.
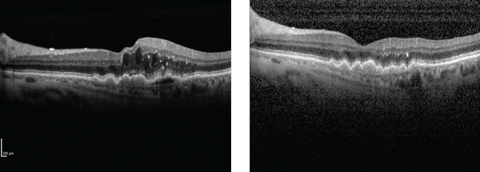  |
| Wet AMD patient before (left) and after (right) treatment with Lucentis. The PDS hopes to maintain clinical improvements of this kind while reducing the treatment burden. Click image to enlarge. |
Different Approaches
Many other methods of reducing anti-VEGF treatment burden have been investigated. Some have failed to deliver enough promising clinical data for development to continue; others remain in testing.
One promising but ultimately disappointing effort involved looking beyond the VEGF pathway by targeting platelet-derived growth factor (PDGF), which recruits pericytes to aid formation of the neovascular matrix in wet AMD and other forms of angiogenesis. Researchers hoped to achieve an additive effect by pairing an anti-PDGF agent with conventional anti-VEGF therapy. This concept was studied for both ranibizumab in the Fovista trials and aflibercept (Eylea, Regeneron) in the Capella trial; unfortunately, Phase III and II trials respectively found that neither resulted in additional benefit to monotherapy with anti-VEGF.12
The topical route has also been explored. Squalamine, a small-molecule drug that targets both VEGF and PDGF, was investigated in the Phase II IMPACT study. This prospective randomized trial compared the experimental drug given topically BID in conjunction with routine ranibizumab injections vs. ranibizumab monotherapy. Though it showed encouraging results, a Phase III study of the drug published in January of this year failed to meet its primary endpoint of mean visual acuity gain at nine months.13,14
An approach actively under review involves inhibition of tyrosine kinase, a cell-signaling molecule that triggers vascular endothelial cell proliferation. Efforts seek to deliver a tyrosine kinase inhibitor (TKI) using either gene therapy or oral administration. Genzyme and Adverum Biotechnologies are each studying a promoter gene packaged in an adenoviral vector that causes cells to produce sFlt01, a new chimeric protein that is a tyrosine kinase inhibitor. Genzyme’s drug is administered by intravitreal injection while Adverum’s is delivered by subretinal injection during vitrectomy surgery. Both have shown positive early results.15-17 An oral TKI drug (Tyrogenex) has also been investigated in preliminary trials as another potential way to improve outcomes in patients treated with ranibizumab.15,18
Researchers are evaluating numerous other receptors throughout the AMD pathophysiological cascade as potential targets in hopes of extended therapy beyond VEGF-based efforts. They are also investigating alternative forms of drug delivery for both established and investigational molecules.
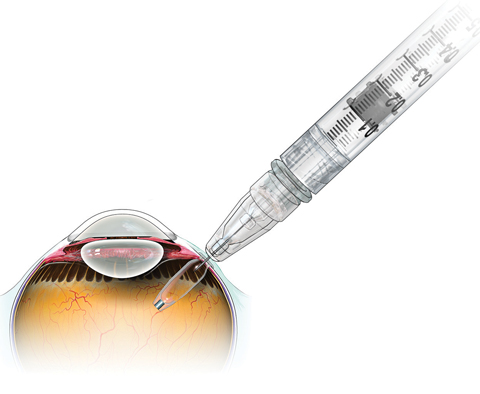 |
| After the device releases all of its initial supply in the reservoir, retina specialists can reload it in the office with a custom needle that is part of the system. This approach is expected to cut the number of injections down from current levels. Click image to enlarge. |
Time to Evolve
The development of anti-VEGF for AMD in 2005 was revolutionary, a true “sea change” that radically reshaped the delivery of care and patient outcomes. But, more than a decade on, the weaknesses of the current model are increasingly evident. The R&D community is now engaged in a global effort to find the next big breakthrough that will maintain acuity gains for patients with greater predictability while reducing the treatment burden and associated costs. In the absence of another revolution, incremental gains are always welcome.
While current studies of the Port Delivery System focus on ranibizumab administration in patients with wet AMD, the underlying technology is a platform that can be retooled for other clinical applications. The volume of the reservoir and the elution rate of drug release can be modified to suit different sizes and types of molecules. Thus, the PDS technology could unlock new treatment options for wet AMD patients as well as others, while also helping physicians and healthcare systems worldwide.
Dr. Loewenstein is a professor of ophthalmology and deputee dean of the medical school at the Sackler Faculty of Medicine, Tel Aviv University, and chair of the department of ophthalmology at the Tel Aviv Sourasky Medical Center.
1. Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153:209-13. |

