As optometrists, we see patients of all walks of life and with many different conditions. Although patients usually present for specific visual and ocular issues, some may present with non-specific and vague concerns not directly linked with a refractive condition; rather, they are secondary to a neurological systemic issue. Because the eye is derived from neuroectoderm and is an extension of the central nervous system (CNS), many conditions, such as Parkinson’s, Alzheimer’s and multiple sclerosis (MS), can have early—even pre-clinical—ocular signs. New research is beginning to build a case for early detection, even from the optometrist’s chair. Here, we discuss these three common neurological conditions and the ocular manifestations that may present during an exam.
An Eye on Diagnostics
Discovering neurodegenerative disorders early can be a challenge, given the expensive and relatively unreliable diagnostic tests it requires. Moreover, no noninvasive measures exist to accurately diagnose preclinical neurodegenerative conditions. Traditionally, a functional magnetic resonance imaging (fMRI) is required to properly identify early disease.1
However, evidence now suggests that ocular technologies can help, given that the cerebral cortex and retina share many features, including anatomical structure, vascular supply and the blood/tissue barrier. Technologies such as optical coherence tomography (OCT), blue light autofluorescence and scanning laser ophthalmoscopy are showing promise in detecting many early neurological diseases.2 The added benefit is that these are noninvasive and cost-effective tools.
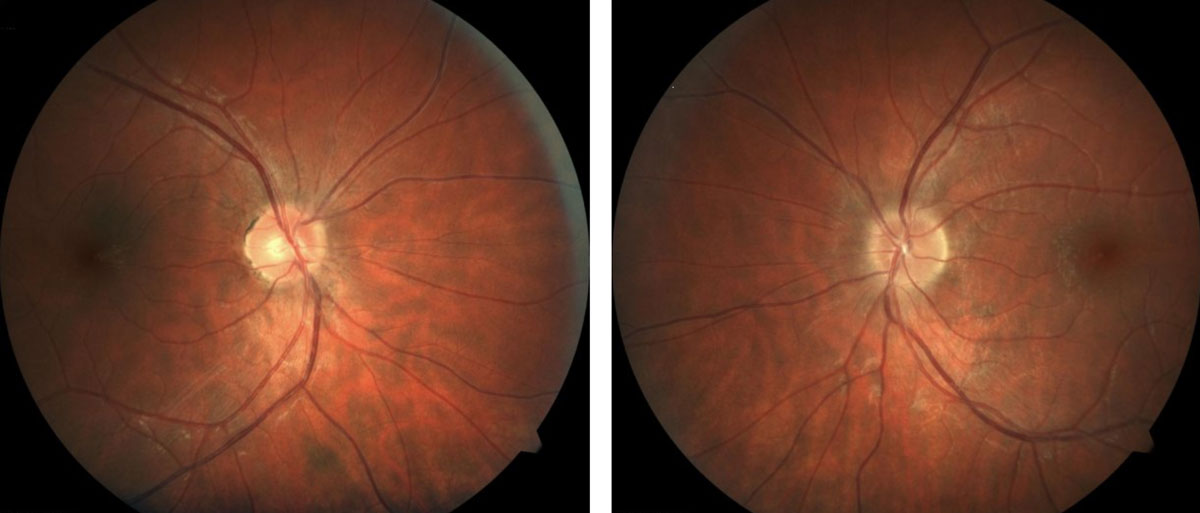 |
| Patients with MS may present with retinal findings such as irregular or indistinct optic nerve margins. Click image to enlarge. |
Parkinson’s Disease
A hallmark of this condition, reduced dopamine production, can cause significant complications in the eye. Patients with Parkinson’s disease may experience reduced contrast sensitivity, for example, and researchers speculate that the lateral inhibition of dopamine in the retina is to blame, ultimately resulting in the global loss of dopamine receptors.3 This leads to visual hallucinations that, with the right patient/caregiver questions, might come up during an ocular exam.4
Recent studies found other local metabolic and hisopathologic changes in the primary visual cortex in patients that have early Parkinson’s disease as well, leading to visual dysfunction, pupil abnormality, lens opacity and retinal neuronal loss and dysfunction.1,5
One of the most clinically relevant findings in early Parkinson’s disease, from an ocular standpoint, is retinal thinning. Researchers first used OCT to find circumpapillary retinal nerve fiber layer (RNFL) thinning in Parkinson’s patients in 2004, noting that 70% of Parkinson’s patients in the study had an inferotemporal RNFL thickness less than 153µm, while all control measurements were above this threshold.6 A 2018 study pinpointed retina thinning to the temporal and inferior 2.22mm sectors and found patients with the thinnest retinas also had the severest disease.7 Since then, research has shown that Parkinson’s disease may be identified early by a thin macular ganglion cell-inner plexiform (GC-IPL) complex on OCT, especially in the parafoveal region.2,4,9-11
Tears Tell The StoryA series of studies evaluated possible CNS disease biomarkers in tears and the ocular surface. The researchers found a decrease in expression of amyloid precursor protein as well as beta amlyoid protein degradation in corneal fibroblasts in Alzheimer’s patients. Parkinson’s patients showed a decrease in blink rate, a decrease in corneal sensitivity, increased expression of specific tear proteins and, ultimately, ocular surface disease.1 Another study found that tear levels of alpha-synuclein may be able to discriminate between patients with Parkinson’s disease and healthy controls, noting that reflex tear alpha-synuclein levels demonstrated a greater increase and sensitivity in distinguishing Parkinson’s patients from healthy controls than basal tears.2 In addition, differences in lactoferrin of reflex tears, not seen in basal tears, may offer a more reliable and sensitive source of biomarkers for Parkinson’s disease.2
|
OCT-angiography (OCT-A) is another promising imaging tool that can measure retinal capillary complexes—a decrease of which has been linked to oxidative stress observed in the Parkinson’s disease process.2,11,12,13-15 In vivo, OCT-A analyzes both the retina and choroidal microvasculature by high speed B-scans. It plots high, slow or no flow zones that indicate areas of abnormal blood flow, possibly confirming decreased retinal microvascular density observed in Parkinson’s patients.16 For example, both the lower retinal capillary quantity and the lower densities of these vascular complexes may be objective biomarkers for Parkinson’s disease.2,11-14
Several other ocular findings have been implicated in Parkinson’s disease, including abnormal electroretinogram responses and reduced motion perception in patients with mild cognitive disorders.17
In addition, patients with Parkinson’s show an increase in alpha-synuclein in both the axons and dendrites of ganglion cells, resembling that of lewy bodies in the brain, even before clinical signs of disease are noted.17 Early animal models show retinal imaging can measure expression of this protein, suggesting its clinical utility in tracking the disease.18
Another protein, the GPR109A receptor, is upregulated in Parkinson’s and can be found in the retinal pigment epithelium.19 Because GPR109A has a high affinity for niacin, which is necessary for dopamine production, researchers speculate that niacin supplementation can provide a potential treatment option for Parkinson’s disease.19
Alzheimer’s Disease
Researchers have identified several possible biomarkers in Alzheimer’s disease, including a significant increase in amyloid plaque deposits in the retina. In addition, investigators consider an increase in drusen a precursor to Alzheimer’s disease. Another biomarker is tau, and an abnormal phosphorylated tau accumulates in the ganglion cell layer in this disease process.20-23 On imaging, researchers found significantly diminished tau protein at the retinal ganglion cell layer at the optic nerve.
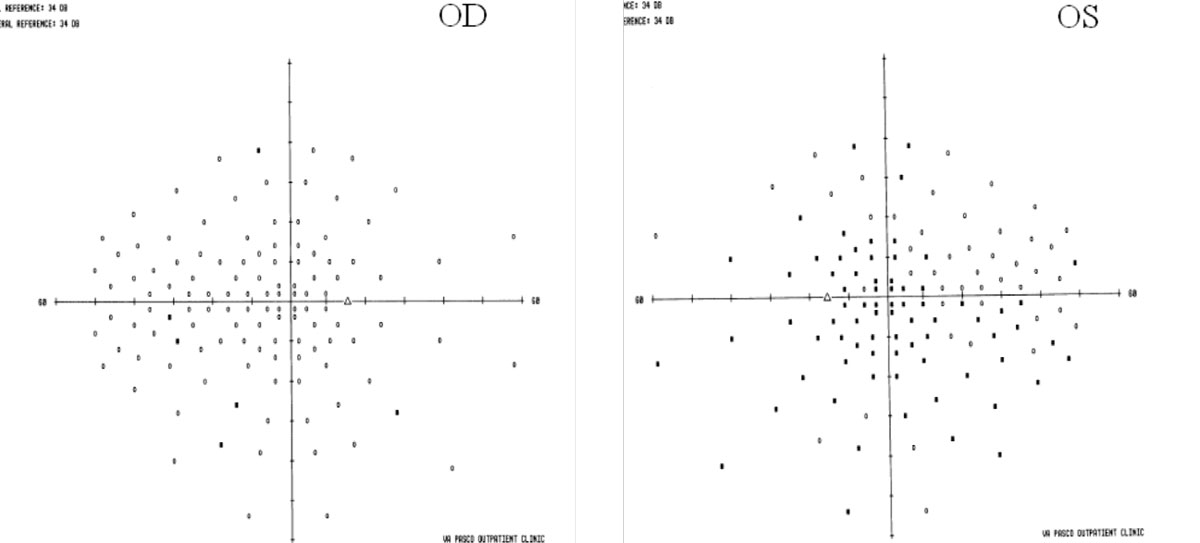 |
| In MS, visual fields may show areas that aren’t a clear as others. Click image to enlarge. |
An increase in these biomarkers increases local inflammation and ultimately disrupts the function of the retinal ganglion cells.24
Although these early retinal findings occur in mild Alzheimer’s disease, they are hard to detect prior to the mild cognitive decline with current noninvasive technology.
Beyond biomarkers, studies have identified marked ocular changes in Alzheimer’s patients, including decreased RNFL thickness and volume, retinal ganglion cell degeneration and reduced retinal blood flow associated with vascular changes observed on OCT. Specifically, only the outer photoreceptor layer shows statistically significant thinning in early Alzheimer’s patients.25
Vascular findings, observed by OCT-A, include enlarged foveal avascular zone, reduced vessel branching, tortuosity and density, increased retinopathy, reduced blood flow, pulsatility, pulse pressure and choroidal changes. Researchers note that the choroid is the thinnest nasally to the macula and thickest temporally to the macula and within the macula.26-29 Clinicians should keep Alzheimer’s in mind when evaluating patients for glaucoma, the GC-IPL complex specifically is atrophied in early Alzheimer’s disease, possibly confounding the diagnosis.26-29
A Mixed Bag
There are many overlapping ocular findings between various neurodegenerative conditions and even other ocular conditions, further complicating accurate diagnosis of a specific disease entity. For example, the saccade velocities and accuracy decrease in patients with motor dysfunction secondary to Parkinson’s, Alzheimer’s and other neurodegenerative conditions, leading to significant overshoot or undershoot.21 The activation of microglia causes an increase in inflammatory cytokines and, ultimately, impaired brain and retinal function as seen with both Parkinson’s and Alzheimer’s patients.30
Thus, while a promising diagnostic finding, GC-IPL thinning on OCT cannot help clinicians differentiate between the many possible etiologies.2,8-13
Despite the growing body of literature supporting the role of the eye in these CNS diseases, Parkinson’s disease and Alzheimer’s disease remain in neurology’s domain, and clinicians who suspect either of them should refer patients appropriately.
The Many Phases of MSAlso called disseminating sclerosis, MS has myriad variations, all of which have the similar signs of demylenation that occur in a waxing and waning manner. A patient often has an initial bout where inflammation and demylenation first occur. This is called the clinically isolated syndrome (CIS), which is considered a precursor to the eventual diagnosis of MS.34,36 The most common variation of MS is called relapsing-remitting (RRMS). This is commonly noted to have episodes where the disease process activates and is followed by a remission of the inflammation and symptoms. These activations, or relapses, can result in a near complete return to normalcy. At times, however, the recovery may not result in a return to baseline; rather, the patient has an incomplete recovery. From this new level there can be further relapses and remissions that continue to demylenate and damage the CNS. This is the hallmark of progression with this variation. RRMS is responsible for nearly 85% of MS cases.36 There are also two types that are labeled as progressive: primary and secondary. Primary progressive is characterized by progression that does not have remissions that return to a level close to baseline. Though it may have instances of remission, these instances result with stability rather than regression. Secondary progressive (SPMS) is often preceded by a designation of RRMS. This is because there is an initial phase similar to the step gradation noted in RRMS. However, there is a more continual and gradual increase in degradation from the disease in SPMS, whereby the trend continues to worsen with less improvement. |
MS: A Clinical Perspective
Unlike Parkinson’s and Alzheimer’s, MS is a condition that optometrists may help to diagnose in their chair. The condition has myriad ocular findings that are precursors to the disease and are often the first symptoms (see, “The Many Phases of MS”).
In our clinical experience, the main ocular symptoms are color vision abnormalities, blurred vision and general asthenopia.31 During confrontation fields, clinicians may note areas that are not as clear as others in the field and even some loss of visual field due to demyelinating lesions along the visual pathway.32 Extraocular motilities are typically normal in early MS, though there can be jerky movements noted later in the disease process. Nystagmus may also be present.
It is not typical to have full restriction in MS. Pupils may also be atypical. In instances of advanced disease with multiple episodes of optic neuritis, an afferent pupillary defect (APD) is likely, as over time, optic neuropathy develops. In early cases, an APD may also be present, though often the pupils may show an APD in one instance but appear normal immediately after.
Color vision testing may also reveal deficits, often with one eye more affected than the other.31 However, retinal or media issues, such as asymmetric cataracts, may cause false positives. The density loss is due to changes of the optic nerve and includes optic neuritis and, ultimately, optic neuropathy.
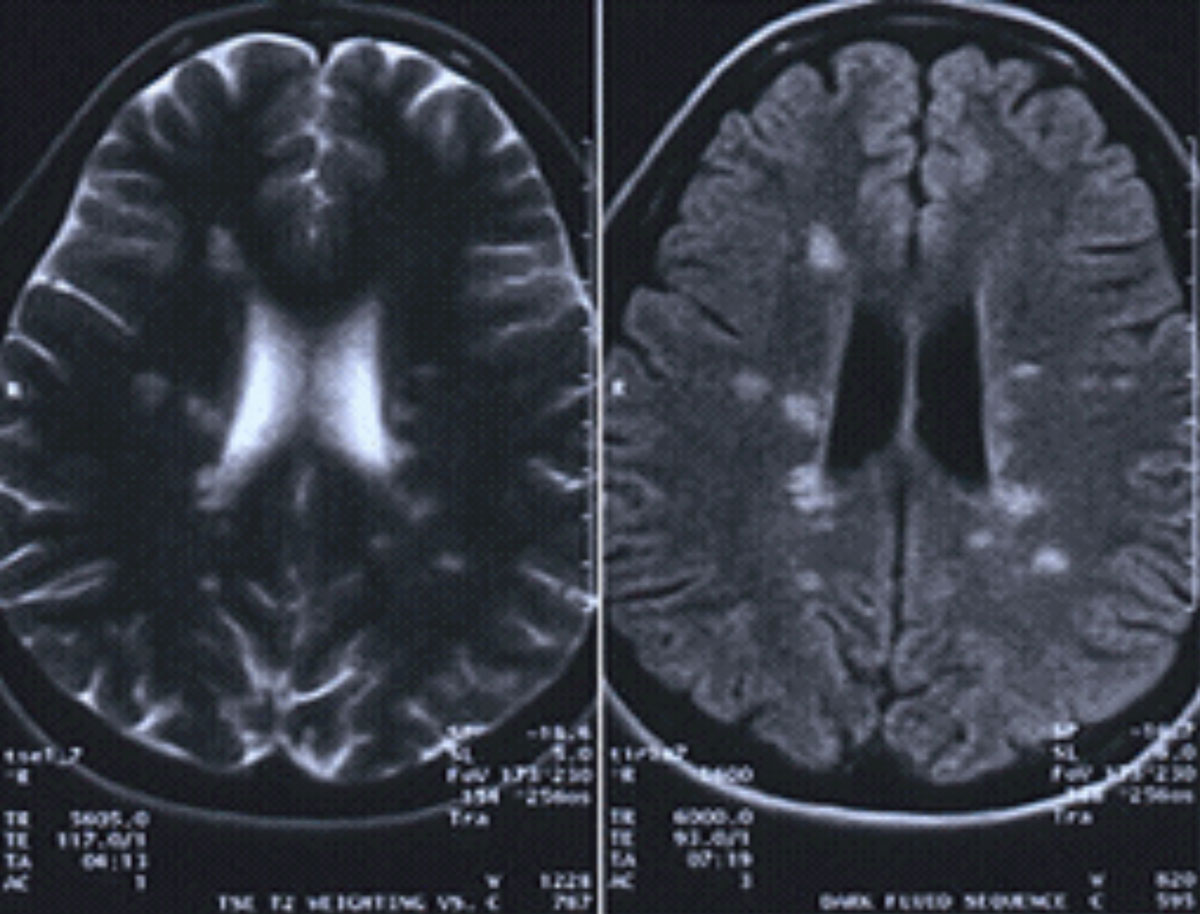 |
| This MRI with and without contrast is positive for MS, showing a lesion located around the ventricles and juxtacortical and in the temporal lobe. Click image to enlarge. |
If after preliminary testing the clinician notes irregular findings, the dilated exam should focus on the optic nerve to assess for irregular or indistinct optic nerve margins, whether 360° or sectoral, which is due to inflammation to the optic nerve. Active cases of unilateral optic neuritis should have MS at the top of the differential diagnosis list, particularly if the patient is in a higher-risk demographic, which includes patients who are between 20 and 40 years of age, female and of Northern European descent.33 If optic neuritis is the suspected diagnosis, consider further consultation with neurology.
Although MS is diagnosed with the patient’s symptomatology, an MRI and gadolinium contrast media is beneficial (unless contraindicated). A positive MRI would show a classic lesion presentation located around the ventricles and juxtacortical and in the temporal lobe.34,35 These lesions are pathognomonic for MS.
Clinical Takeaways
Detecting and managing these neurodegenerative conditions requires a multidisciplinary approach. The recently discovered retinal biomarkers, while promising, are not specific for a definitive neurodegenerative condition. Further study and longitudinal research will hopefully validate a connection with various neurodegenerative conditions.
Until then, optometrists can keep a close eye on the literature and be ready to recognize the possible early signs of CNS disease manifesting in the eye. As the population’s age increases, so does neurodegenerative disease, and optometrists need to stay vigilant to catch it prior to any cognitive decline.
Dr. Suhr is the chief of optometry at the Philadelphia Corporal Michael J. Crescenz VA Medical Center. He is a fellow of the Optometric Retina Society and the American Academy of Optometry. His clinical focus is primarily on retinal disease, glaucoma, neuro-ophthalmic disease and oculosystemic disease.
Dr. Patel is a staff optometrist at the Philadelphia Corporal Michael J. Crescenz VA Medical Center. He is a fellow of the American Academy of Optometry. His main clinical focus is primarily on glaucoma, and he has a deep interest in neuro-ophthalmic disease.
1. Moro, E, Bellot E, Meoni S, et al. Visual dysfunction of the superior colliculus in de novo parkinsonian patients. Ann Neurol. 2020;87(4):533-46. 2. Chrysou A, Jansonius NM, van Laar T. Retinal layers in Parkinson’s disease: A meta-analysis of spectral-domain optical coherence tomography studies. Parkinsonism Relat Disord. 2019;64:40-49. 3. Whitfield WH, Barr GQ, Khayata MJ, et al. Contrast sensitivity visual acuity in REM sleep behavior disorder: a comparison with and without Parkinson disease. J Clin Sleep Med. 2020;16(3):385-88. 4. Kurita A, Koshikawa H, Akiba T, et al. Visual hallucinations and impaired conscious visual perception in Parkinson disease. J Geriatr Psychiatry Neurol. 2019 December 6;891988719892318. 5. Guo L, Normando EM, Shah PA, et al. Oculo-visual abnormalities in Parkinson’s disease: Possible value as biomarkers. Mov Disord. 2018;33(9):1390-1406. 6. Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Research. 2004;44(24):2793-7. 7. Ahn J, Lee JY, Kim TW, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology. 2018;91(11):e1003-12. 8. Lee JY, Ahn J, Oh S, et al. Retina thickness as a marker of neurodegeneration in prodromal lewy body disease. Mov Disord. 2020;35(2):349-54. 9. Tugcu B, Melikov A, Yildiz GB, et al. Evaluation of retinal alterations in Parkinson disease and tremor diseases. Acta Neurologica Belgica. 2020;120(1):107-13. 10. Wong BM, Cheng RW, Mandelcorn ED, et al. Validation of optical coherence tomography retinal segmentation in neurodegenerative disease. Tran Vis Sci Technol. 2019;8(5):6. 11. Murueta-Goyena A, Del Pino R, Reyero P, et al. Parafoveal thinning of inner retina is associated with visual dysfunction in Lewy body diseases. Mov Disord. 2019;34(9):1315-24. 12. Mailankody P, Lenka A, Pal PK. The role of optical coherence tomography in parkinsonism: a critical review. J Neurol Sci. 2019;403:67-74. 13. Esquiva G, Hannibal J. Melanopsin-expressing retinal ganglion cells in aging and disease. Histol Histopathol. 2019;34(12):1299-1311. 14. Shi C, Chen Y, Kwapong WR, et al. Characterization by fractional dimension analysis of the retinal capillary network in parkinson disease. Retina. 2020;40(8):1483-91. 15. Martín-Nieto J, Uribe ML, Esteve-Rudd J, et al. A role for DJ-1 against oxidative stress in the mammalian retina. Neurosci Letters. 2019;708:134361. 16. Kwapong WR, Ye H, Peng C, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Opthalmol Vis Sci. 2018;59(10):4115. 17. Ortuño-Lizarán I, Beach TG, Serrano GE, et al. Phosphorylated α-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov Disord. 2018;33(8):1315-24. 18. Price DL, Koike MA, Khan A, et al. The small molecule alpha-synuclein misfolding inhibitor, NPT200-11, produces multiple benefits in an animal model of Parkinson’s disease. Sci Rep. 2018;8(1):16165. 19. Wakade C, Chong R. A novel treatment target for Parkinson’s disease. J Neurol Sci. 2014;347(1–2):34-38. 20. Kile S, Au W, Parise C, et al. Reduction of amyloid in the brain and retina after treatment with IVIG for mild cognitive impairment. Am J Alzheimers Dis Other Demen. 2020;35:1533317519899800. 21. Benvenutto A, Guedj E, Felician O, et al. Clinical phenotypes in corticobasal syndrome with or without amyloidosis biomarkers. J Alzheimers Dis. 2020;74(1):331-43. 22. Papadopoulos Z. Neovascular age-related macular degeneration and its association with Alzheimer’s disease. Curr Aging Sci. January 29, 2020. [Epub ahead of print]. 23. Chintapaludi SR, Uyar A, Jackson HM, et al. Staging Alzheimer’s disease in the brain and retina of B6.APP/PS1 mice by transcriptional profiling. J Alzheimers Dis. 2020;73(4):1421-34. 24. Hart NJ, Koronyo Y, Black KL, Koronyo-Hamaoui M. Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta Neuropathologica. 2016;132(6):767-87. 25. Jáñez-Escalada L, Jáñez-García L, Salobrar-García E, et al. Spatial analysis of thickness changes in ten retinal layers of Alzheimer’s disease patients based on optical coherence tomography. Sci Rep. 2019;9(1):13000. 26. Alber J, Goldfarb D, Thompson LI, et al. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimers Dementia. 2020;16(1):229-43. 27. Asanad S, Ross-Cisneros FN, Barron E, et al. The retinal choroid as an oculovascular biomarker for Alzheimer’s dementia: A histopathological study in severe disease. Alzheimers Dementia. 2019;11:775-83. 28. Almeida ALM, Pires LA, Figueiredo EA, et al. Correlation between cognitive impairment and retinal neural loss assessed by swept-source optical coherence tomography in patients with mild cognitive impairment. Alzheimers Dementia. 2019;11:659-69. 29. Chiquita S, Campos EJ, Castelhano J, et al. Retinal thinning of inner sub-layers is associated with cortical atrophy in a mouse model of Alzheimer’s disease: a longitudinal multimodal in vivo study. Alzheimers Res Thera. 2019;11(1):90. 30. Ramirez AI, de Hoz R, Salobrar-Garcia E, et al. The role of microglia in retinal neurodegeneration: Alzheimer’s disease, Parkinson, and Glaucoma. Front Aging Neurosci. 2017;9:214. 31. Piro A, Tagarelli A, Nicoletti G, et al. Impairment of acquired color vision in multiple sclerosis: an early diagnostic sign linked to the greatness of disease. Int Ophthalmol. 2019;39(3):671-76. 32. Costello F. Vision disturbances in multiple sclerosis. Semin Neurol. 2016;36(2):185-95. 33. Kamm CP, Uitdehaag BM, Polman CH. Multiple sclerosis: current knowledge and future outlook. Eur Neurol. 2014;72(3-4):132-41. 34. The Radiology Assistant: Multiple Sclerosis. https://radiologyassistant.nl/neuroradiology/multiple-sclerosis. May 13, 2013. Accessed September 16, 2020. 35. Wilson JA, Islam O. brain imaging in multiple sclerosis: practice essentials, computed tomography, magnetic resonance imaging. Medscape. https://emedicine.medscape.com/article/342254-overview. March 27, 2019. Accessed September 16, 2020. 36. Ruiz F, Vigne S, Pot C. Resolution of inflammation during multiple sclerosis. Semin Immunopathol. 2019;41(6):711-26. |

