24th Annual Glaucoma ReportFollow the links below to read other articles from annual update on glaucoma: MIGS Madness: An Atlas of Options A Guide to Applying IOP-lowering Drops Glaucoma: Lifestyles of the Antioxidant Rich and Famous (Earn 2 CE Credits) |
Glaucoma treatment has undergone significant changes in both pharmaceutical and surgical arenas over the last few years. New topical glaucoma drugs with multiple mechanisms of action are helping to lower intraocular pressure (IOP), but patient adherence, ocular toxicity and lifestyle issues continue to be detriments. Minimally invasive glaucoma surgeries (MIGS), targeting mild-to-moderate glaucoma, are providing ophthalmologists with a wide array of devices to lower IOP. Technological advances allow for earlier detection, yet patients progress and severe glaucoma continues to exist. In these cases, traditional incisional surgeries are necessary to prevent debilitating vision loss.
Optometrists must understand why these procedures are necessary, how they are performed and our roles in the pre- and post-op care of traditional incisional surgeries.
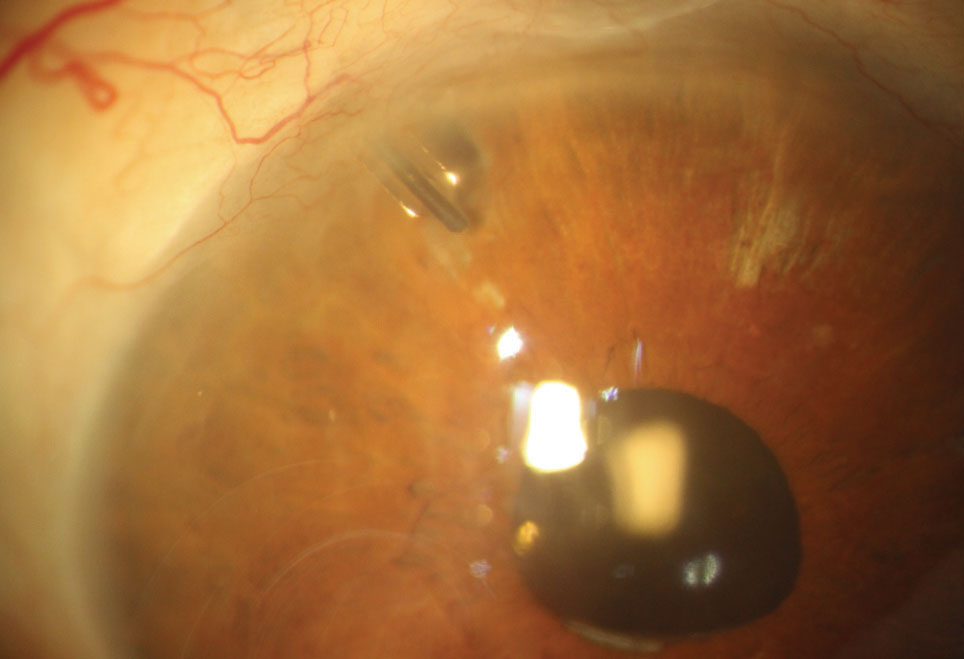 |
| Fig. 1. Here you can see a Baerveldt aqueous tube shunt with the nylon “ripcord” suture visible within the lumen of the tube shunt. If additional IOP lowering is needed, the suture can be removed to increase flow through the tube shunt. |
Preoperative Considerations
Surgical intervention is usually not the first choice for treating a glaucoma patient. Topical glaucoma drops or selective laser trabeculoplasty are initially good options for high-risk glaucoma suspects and both mild and moderate glaucoma patients. However, when a patient displays above-target IOP and VF progress and when OCT shows retinal nerve fiber layer (RNFL) thinning and topical medications are maxed out, clinicians should start a conversation about surgery. The emergence of MIGS has given surgeons an option for patients with mild-to-moderate glaucoma. However, when these procedures are not effective enough, a more aggressive approach is necessary.
The decision on what type of filtration surgery often comes down to surgeon preference and the procedure they are most comfortable with. As this article will show, all of the procedures have the ability to effectively lower IOP. At times, the anatomy of the eye can dictate the decision as well. If the patient has a small globe or a particularly narrow palpebral fissure, placing an aqueous tube shunt can be difficult and a trabeculectomy may be optimal. As the primary eye care provider, it’s the optometrist’s responsibility to remind patients that these surgeries have an extended healing period. It is not uncommon for the patient to have a foreign body sensation or discomfort in the eye for at least one week. It can take months before IOP stabilizes, and at times can fluctuate from too low to too high. If expectations are set before surgery, the postoperative period for both the OD and patient will go smoother.
Devices and Procedures
Aqueous tube shunts divert aqueous humor from the anterior chamber to an external reservoir. A fibrous capsule forms about one month after implantation over the plate of the tube shunt to regulate flow.
Tube shunts come in a variety of sizes, materials and designs. Some familiar ones include the Molteno implant (Katena), the Baerveldt implant (AMO/Johnson & Johnson Vision) and the Ahmed glaucoma valve (New World Medical).
The Molteno and Baerveldt implants are nonvalved devices and, until the fibrous capsule forms, have no way of controlling aqueous flow, which can lead to early postoperative hypotony (Figure 1). For that reason, the devices are partially occluded with a nylon suture and tied off with a four-to six-week dissolvable vycryl suture. This technique causes postoperative IOP to remain unchanged, requiring patients to continue all of their preoperative glaucoma medications until the fibrous capsule forms to regulate aqueous flow and the suture dissolves. The nonvalved devices are a good choice for glaucoma patients who do not need immediate IOP control. The surgeon has the ability to attempt to predict or guide the postoperative IOP with a Baerveldt implant by selecting different sizes.
The Ahmed glaucoma valve provides a more immediate IOP response. The valve mechanism consists of multiple thin membranes that are pretensioned to open and close in response to IOP variations, in the range of 8mm Hg to 12mm Hg.1 A lower rate of hypotony is seen with Ahmed glaucoma valves compared with Baerveldt implants.2
Implanting these devices requires a conjunctival incision to create a conjunctival flap between two recti muscles, typically in the superotemporal quadrant.3 Tenon’s capsule is dissected from the episclera and episcleral vessels are gently cauterized. The implant is placed 8mm to 10mm from the limbus and sutured to the sclera.3 The drainage tube is trimmed to 2mm to 3mm insertion and bevel cut to an angle of 30 degrees to allow access to the anterior chamber. A 22g to 23g needle is used to create a tract for tube insertion in the anterior chamber.3
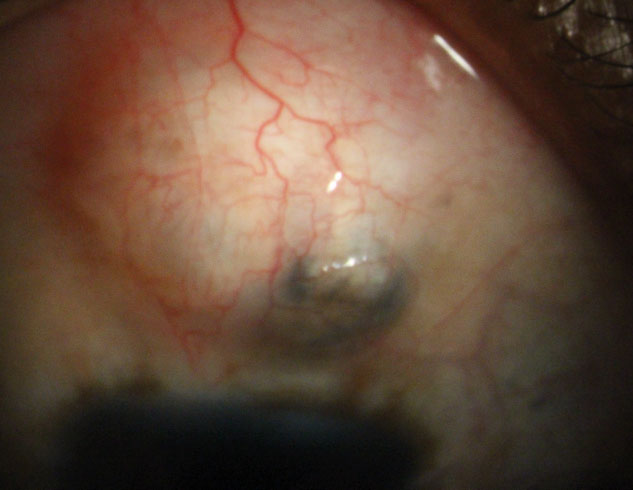 |
| Fig. 2. The filtering bleb of a patient with aphakic glaucoma and nystagmus. Note the area of pigment in the bleb secondary to iris pigment liberation. |
Trabeculectomy
This filtering surgery creates an opening into the anterior chamber from underneath a scleral flap (Figure 2). The opening allows aqueous to flow out of the eye, into the subconjunctival space and creates a filtering bleb.4
According to a 2011 survey by the American Glaucoma Society, trabeculectomy started to decrease in popularity between 1996 and 2008.5 Concerns with bleb-related complications has lead to an increased use of tube shunts. The survey reveals members prefer trabeculectomy with mitomycin C (MMC) in 74% of patients compared with 11% placement of tube shunts in 2008.5 In 2016, the survey was repeated, and tube shunt surgery increased to 23% compared with a decrease of 59% use of trabeculectomy with MMC.6
The surgical technique begins with a creation of a superior fornix-based conjunctival flap, and MMC is applied to the bare sclera for one to four minutes. MMC is an antimetabolite agent that prevents subconjunctival fibrosis and subsequent failure of the trabeculectomy. Next, a partial thickness scleral flap is created, the anterior chamber is entered with a thin metal probe called a stylet and a peripheral iridectomy (PI) is performed. The final steps of the procedure involve the scleral flap being sutured and a bleb being formed by suturing the conjunctiva to the limbus.
Express mini glaucoma shunt (Alcon) is a device with characteristics of an aqueous tube shunt and a trabeculectomy (Figure 3). It is a stainless steel 3mm biocompatible device with an external diameter of 400µm.7 It is non-valved, with a 50µm lumen and an external disc or plate at one end and a spur-like extension on the other end to prevent extrusion.7 The device shunts aqueous from the anterior chamber to a subconjunctival reservoir similar to a trabeculectomy, but without the removal of scleral or iris tissue.8
The surgeon makes a superior fornix-based conjunctival incision to allow the creation of a scleral flap in the same manner as a trabeculectomy. A temporal paracentesis wound is created through the cornea and MMC is applied to the bare sclera. The scleral flap is lifted and a 26g needle is inserted into the anterior chamber to allow access for the device. It comes preloaded on an injector and its lumen gains access to the chamber through the wound created by the needle. The plate of the device is placed flush with the scleral bed and the scleral flap is sutured into place. One to three releasable sutures are placed and can be removed depending on the aqueous flow required. The conjunctiva is closed and, using the paracentesis wound, the bleb is inflated with balanced salt solution.8-10
 |
| Fig. 3. The lumen of an Express mini glaucoma shunt visible in the anterior chamber. |
In The Lit
One major analysis of the efficacy and safety of tube shunt surgery and trabeculectomy, the Tube Vs. Trabeculectomy (TVT) study, was designed prospectively over a five-year period.11 It looked at 212 patients with uncontrolled glaucoma who had previously undergone cataract extraction, had failed filtering surgery or both.11 Patients were randomized to receive either a Baerveldt glaucoma implant or a trabeculectomy with MMC.11 The goal of the study was to provide information to assist with surgical decisions between tube shunt surgeries versus trabeculectomy with MMC.11
The degree of IOP reduction was effective and similar between treatment groups at five years. The baseline IOP was 25.1mm Hg +/-5.3mm Hg in the tube group and 25.6mm Hg +/-5.3mm Hg in the trab group. At five years, IOP was 14.4mm Hg +/-6.9mm Hg in the tube group and 12.6mm Hg +/-5.9mm Hg in the trabeculectomy group.
The majority of patients enrolled in the TVT study had advanced glaucoma; thus, many had initiated near maximal medical therapy. The researchers noted a significant reduction in medication use in both groups, but no significant difference in mean number of medications between the groups at five years. Baseline medications were 3.2 +/-1.1 in the tube group and 3.0 +/-1.2 in the trabeculectomy group. At five years, mean number of medications was 1.4 +/-1.3 in the tube group and 1.2 +/-1.5 in the trab group.
The cumulative probability of failure at five years was 29.8% in the tube group and 46.9% in the trabeculectomy group. Failure included patients with persistent hypotony, reoperation for glaucoma, or loss of light perception vision. The higher failure rate of the trab group at five years was consistent with data at one year and three years.12,13
The TVT study does not show a clear superiority of one glaucoma procedure over the other, but a shift in the use of tube shunts has occurred since 2008.5,6 One reason may be the increased failure rates of trabeculectomy surgery compared with tube shunt surgery.5,6
The Primary Tube vs. Trabeculectomy (PTVT) study is a randomized clinical trial comparing the safety and efficacy of tube shunt surgery and trabeculectomy with MMC in eyes without prior ocular surgery.14 The study is ongoing. Similar to the TVT study, the PTVT study goal is to assist with surgical decisions between tube shunt surgeries and trabeculectomy with MMC.14
The degree of IOP reduction was effective and similar between treatment groups at one year. Baseline IOP was 23.3mm Hg +/-4.9mm Hg in the tube group and 23.9mm Hg +/-5.7mm Hg in the trab group. At one year, IOP was 13.8mm Hg +/-4.1mm Hg in the tube group and 12.4m Hg +/-4.4mm Hg in the trabeculectomy group. One year IOP reduction results are similar to what was found in the five-year results of the TVT study, showing the effective and powerful IOP lowering ability of both procedures.11
Medication reduction occurred in both groups, but significantly greater use of medication was seen in the tube shunt group vs. the trabeculectomy group at one year. Baseline medications were 3.1 +/- 1.1 in the tube group and 3.2 +/-1.1 in the trabeculectomy group. At one-year follow-up, mean number of medications was 2.1 +/-1.4 in the tube group and 0.9 (+/-1.4) in the trabeculectomy group.
The cumulative probability of failure at one-year follow-up was 17.3% in the tube group and 7.9% in the trabeculectomy group. The result of failure rates is opposite to what was seen in the TVT study five-year results.11 Ultimately, failure rates will depend on follow-up for the PTVT study.
Similar to the TVT study, PTVT does not demonstrate a clear superiority for one procedure over the other. In contrast to the TVT study, PTVT has made a case that not only is trabeculectomy efficacious in lowering IOP, but demonstrates a lower failure rate than tube shunt surgery at one year. A longer follow-up period is necessary to fully compare the two studies.
Express mini glaucoma shunt vs. trabeculectomy. The Express shunt was designed to be a simpler aqueous filtration device with the efficacy of a trabeculectomy, with reduced rates of complications. Multiple studies have reported no significant difference in IOP reduction between the procedures.15-18 The Express shunt has been shown to have reduced rates of early postoperative hypotony, thought to be because of uniform filtration through the lumen of the device.15,16,18,19 There have also been studies showing that less inflammation occurs postoperatively with the Express shunt as no iridotomy is performed.15,16,18,19 Long-term hypotony, bleb morphology, visual acuity and bleb-related complications have been shown to be similar to trabeculectomy.15-19
With many studies showing equal efficacy and safety of Express shunt and trabeculectomy, economic differences have been a topic often discussed. One study reported a 3.5x higher disposable cost for Express vs. trabeculectomy.20
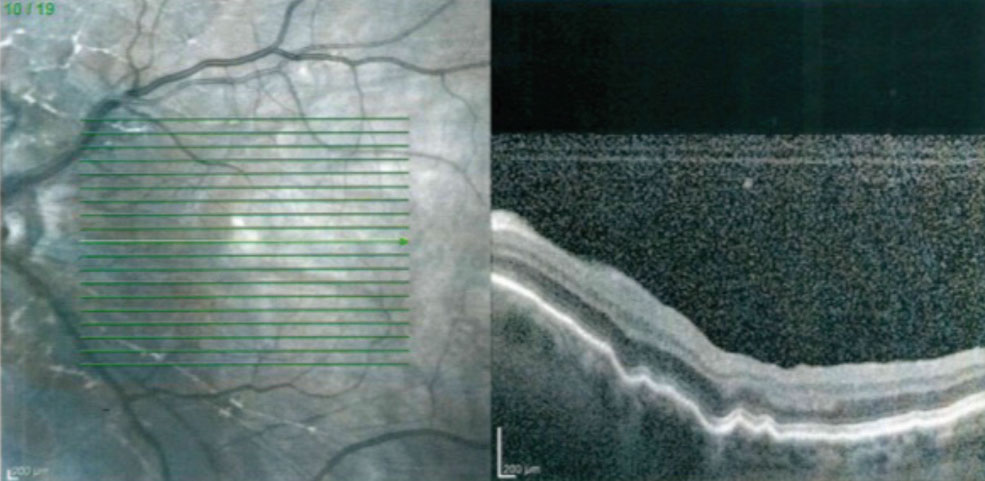 |
| Fig. 4. The macular OCT of a patient with a Express mini glaucoma shunt and hypotony maculopathy. Note the choroidal folds and retinal striae. |
Postoperative Considerations and Complications
Trabeculectomy’s and Express shunts’ typical post-op care involves visits according to the IOP, anterior chamber depth and bleb characteristics. A low-lying diffuse bleb with minimal vascularity, cystic changes, low teens IOP, formed anterior chamber and a Seidel-negative conjunctival closure is an ideal bleb.21,22 The most common complications occur due to changes in bleb structure. These complications include hypotony, bleb leaks and blebitis.21,22
Hypotony can present secondary to a conjunctival wound leak in the early postoperative period. The key finding is a low IOP—less than 5mm Hg—without a visible bleb. A Seidel’s test will localize the site of the leak, and conservative treatment is initially recommended. Conservative treatment involves placing a bandage contact lens, aqueous suppressant drops and the initiation of antibiotic drops. If the eye fails to respond, a referral back to the surgeon is warranted for possible surgical intervention.23
Hypotony that is present for three months or longer is classified as chronic hypotony. Chronic hypotony can lead to hypotony maculopathy, which is associated with a decrease in visual acuity, choroidal folds, retinal striae and the absence of edema (Figure 4).24,25 Chronic hypotony is a challenge to manage as surgical intervention (bleb size reduction by cryotherapy, compression sutures, closing of the scleral flap or applying a scleral patch graft) results are variable and, at times, can lead to unsafe levels of IOP elevation.26-28
The use of MMC can cause thin-walled blebs, increasing the risk of a bleb leak and subsequent hypotony, blebitis or endophthalmitis (Figure 5).29 A bleb leak is detected by using a fluorescein strip and cobalt blue illumination. The fluorescein strip is applied to the bleb; if a leak is present, subtle unstained aqueous will be seen flowing from the bleb with dark green fluorescein stained tear film surrounding it.
Treatment should be conservative, with the initiation of aqueous suppressants, topical antibiotic, application of a bandage contact lens or use of an amniotic membrane graft.30 If conservative treatment does not resolve the leak, a referral back to the surgeon is best, and closure of the leak by cyanoacrylate glue, fibrin tissue glue or surgical revision can be attempted.31-33
Bleb-related ocular infections such as endophthalmitis and blebitis have been reported at 2% and as high as 6% to 7.5%.34 The most common symptoms are ocular pain, foreign body sensation, and blurry vision. If an ocular infection is suspected examination of the bleb for a milky white appearance and loss of clarity is critical, as well as a careful examination for any anterior chamber or vitreous involvement.
Cataract Surgery’s WindfallSurgeons have developed many invasive procedures specifically to lower IOP. However, one of the oldest procedures in eye care—in fact, in human history—has always had that effect. Though never designed to lower IOP, cataract surgery alone can reduce it by 16.5%.1 The mechanism is not precisely known, but researchers believe it improves the function of the trabecular meshwork (TM) itself, rather than aqueous access to the TM.2 However, for patients with pre-existing glaucoma, the change is slight at best and can be complicated by prior surgery and elevated IOP at the outset.3,4 Clinicians must carefully manage expectations for cataract patients who already have glaucoma before touting it as a stone that can kill two birds.
|
A blebitis is an infection limited to the filtering bleb and will likely respond well to aggressive antibiotic treatment. If there is involvement of the anterior chamber, vitreous and bleb, the main concern is endophthalmitis. A referral to the surgeon should be made so a fluid tap can be done. Topical, systemic, and, likely, intravitreal injections of antibiotics will be necessary.
Aqueous tube shunt complications share some of the issues encountered with trabeculectomy, but also have some unique issues.
The majority of complications with aqueous tube shunts are hypotony related. Hypotony can occur in the early or late post-op period and with valved or non-valved shunts. On examination, if the anterior chamber is shallow or flat, or choroidal effusions exist, the surgeon will inject a small volume of viscoelastic into the anterior chamber. If hypotony persists after numerous injections, the patient may need tube ligation.
In the TVT study, 6% of patients receiving an aqueous tube shunt had persistent diplopia after three years. Bleb height is one likely reason for diplopia. It is argued that proper placement of the tube plate under the muscles will prevent diplopia. In certain cases of persistent and transient diplopia, spectacle lenses with prism may need to be prescribed to give patients relief.
Other complications may include tube-corneal touch, uncontrolled high IOP, endophthalmitis, corneal edema and tube migration.
In cases of tube-corneal touch, the tube will likely need to be trimmed as corneal endothelial decompensation can occur, along with corneal edema. If the corneal edema persists after the tube is trimmed and a conservative approach of topical steroid is initiated, a partial thickness corneal transplant procedure, such as Descemet’s membrane endothelial keratoplasty or Descemet’s stripping endothelial keratoplasty, may be necessary.
A phenomenon called the ocular hypertensive phase usually occurs one to three months following surgery. It is due to a capsular fibrosis, and treatment is with aqueous suppressants and topical steroids.
Tube migration or tube exposure can lead to endophthalmitis. If the device is partially or completely exposed it may need to be removed to prevent an ocular infection.
Traditional incisional glaucoma surgeries continue to be an indispensable tool in glaucoma, particularly when medications, laser and MIGS fail to lower IOP adequately and stop progression. Although many of these procedures have significant complications that need to be managed in the postoperative period, they also have powerful pressure-lowering abilities.
Dr. Schweitzer practices at Vance Thompson Vision in Sioux Falls, SD, and is an adjunct clinical professor at the Illinois College of Optometry.
1. Coleman A, Hill R, Wilson MR, et al. Initial clinical experience with the Ahmed glaucoma valve implant. Am J Glaucoma. 2014;23(2):e91-e97. 2. Christakis P, Zhang D, Budenz D, et al. Five-year pooled data analysis of the Ahmed Baerveldt comparison study and the Ahmed vs. Baerveldt study. Am J Ophthalmol. 2017;176:118-26. 3. Riva I, Roberti G, Oddone F, et al. Ahmed glaucoma valve implant: surgical technique and complications. Clin Ophthalmol. 2017;11:357-67. 4. Jinza K, Saika S, Kin K, et al. Relationship between formation of a filtering bleb and an intrascleral aqueous drainage route after trabeculectomy: Evaluation using ultrasound biomicroscopy. Ophthalmic Res. 2000; 32:240-3. 5. Desai M, Gedde S, Feuer W, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society in 2008. Ophthalmic Surg Lasers Imaging. 2011;42:202-8. 6. Vinod K, Gedde S, Feuer W, et al. Practice preferences for glaucoma surgery: a survey of the American Glaucoma Society. J Glaucoma. 2017;26:687-93. 7. Nyska A, Glovinsky Y, Belkin M, Epstein Y. Biocompatibility of the Ex-PRESS miniature glaucoma drainage implant. J Glaucoma. 2003;12:275-80. 8. Sarkisian SR. The Ex-Press Mini Glaucoma Shunt: Technique and Experience. Middle East Afr J Ophthalmol. 2009 Jul-Sep;16(3): 134-7. 9. Dahan E, Carmichael T. Implantation of a miniature glaucoma device under a scleral flap. J Glaucoma. 2005;14:98-102. 10. Sarkisian SR. Use of an injector for the Ex-PRESS Mini Glaucoma Shunt. Ophthalmic Surg Lasers Imaging. 2007;38:434-6. 11. Gedde S, Schiffman J, Feuer W, et al. Tube versus trabeculectomy study group. Treatment outcomes in the tube vs. trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153:789-803. 12. Gedde S, Schiffman J, Feuer W, et al. Treatment outcomes in the tube vs. trabeculectomy study. Am J Ophthalmol. 2007;143(1):9-22. 13. Gedde S, Schiffman J, Feuer W, et al. Three-year follow-up of the tube vs. trabeculectomy study. Am J Ophthalmol. 2009;148(5):670-84. 14. Gedde SJ, Feuer WJ, Wei Shi, MS, et al. Treatment outcomes in the primary tube vs. trabeculectomy study after one year of follow-up. Ophthalmology. 2018;125(5):1-14. 15. Maris PJG, Ishida K, Netland PA. Comparison of trabeculectomy with Ex-PRESS miniature glaucoma device implanted under sclera flap. J Glaucoma. 2007;16:14-19. 16. Dahan E, Ben Simon G, Lafuma A. Comparison of trabeculectomy and Ex-PRESS implantation in fellow eyes of the same patient: a prospective, randomized study. Eye. 2012;26:703-10. 17. Seider MI, Rofagha S, Lin SC, et al. Resident-performed Ex-PRESS shunt implantation versus trabeculectomy. J Glaucoma. 2012;21:469-74. 18. Marzette L, Herndon LW. A comparison of the Ex-PRESS mini glaucoma shunt with standard trabeculectomy in the surgical treatment of glaucoma. Ophthalmic Surg Lasers Imaging. 2011;42:453-59. 19. Good T, Kahook M. Assessment of bleb morphologic features and postoperative outcomes after Ex-PRESS drainage device implantation versus trabeculectomy. Am J Ophthalmol. 2011;151:507-13. 20. Valentine J, Zurakowski D, Ayyala R. Comparison of acquisition costs of surgical supplies in different health care systems for cataract and glaucoma procedures. J Glaucoma. 2014;23(6):355-9. 21. Haynes W, Alward W. Control of intraocular pressure after trabeculectomy. Surv Ophthalmol. 199;43:345-55 22. Skuta GL, Parrish RK. Second wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987;32:149-70. 23. Vijaya L, Manish P, Ronnie G, et al. Management of complications in glaucoma surgery. Indian J Ophthalmol. 2011 Jan;59(Suppl1): S131-S140. 24. Detry Morel M, Kittel B. Surface-wrinkling maculopathy as a potential complication of trabeculectomy: a case report. Ophthalmic Surg. 1991;22:38-40. 25. Oyakhire JO, Moroi SE. Clinical and anatomical reversal of long-term hypotony maculopathy. Am J Ophthalmol. 2004;137:935-5. 26. Cohen SM, Flynn HW, Jr, Palmberg PF., et al. 2nd Treatment of hypotony maculopathy after trabeculectomy. Ophthalmic Surg Lasers. 1995;26:435-41 27. Costa VP, Wilson RP, Moster MR, et al. Hypotony maculopathy following the use of topical mitomycin C in glaucoma filtration surgery. Ophthalmic Surg. 1993;24:389-94. 28. Schwartz GF, Robin AL, Wilson RP, et al. Resuturing the scleral flap leads to resolution of hypotony maculopathy. J Glaucoma. 1996;5:246-51. 29. Belyea DA, Dan JA, Stamper RL, et al. Late onset of sequential multifocal bleb leaks after glaucoma filtering surgery with 5-fluorouracil and mitomycin C. Am J Ophthalmol. 1997;124:40-5. 30. Sethi P, Patel RN, Goldhardt R, Ayyala RS. Conjunctival advancement with subconjunctival amniotic membrane draping technique for leaking cystic blebs. Journal of glaucoma. 2016 Feb 1;25(2):188-92. 31. Zalta AH, Wieder RH. Closure of leaking filtering blebs with cyanoacrylate tissue adhesive. Br J Ophthalmol. 1991;75:170-3. 32. Kajiwara K. Repair of a leaking bleb with fibrin glue. Am J Ophthalmol. 1990;109:599-601. 33. Liebmann JM, Sokol J, Ritch R. Management of chronic hypotony after glaucoma filtration surgery. J Glaucoma. 1996;5:210-20. 34. DeBry PW, Perkins TW, Heatley G, et al. Incidence of late-onset bleb relatd complications following trabeculectomy mitomycin. Arch Ophthalmol. 2002;111:1495-503. |

