 |
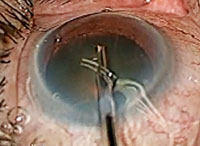
| The Trabectome procedure ablates 60° to 120° of trabecular meshwork tissue. |
 |
|
The iStent is placed in Schlemm’s canal to augment aqueous outflow.
|
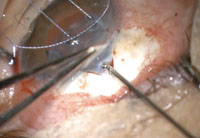 |
| The Express mini-shunt, sometimes considered to be a MIGS procedure, is a safer alternative to trabeculectomy. |
How should ODs approach glaucoma comanagement in the MIGS era? Let’s first review the conventional IOP-lowering modalities, then look at how MIGS fits in.
The Old Standbys
The goal of glaucoma therapy, of course, is to preserve visual function by lowering IOP to a level less likely to produce further damage to the optic nerve, based on many patient-specific factors (e.g., age, ability to tolerate chronic medication use, disease severity and progression). Traditionally, we have managed patients with topical or oral therapy, trabeculoplasty, and incisional glaucoma surgeries (trabeculectomy or drainage devices).
• Medical therapy. Topical anti-hypotensive agents remain the first-line therapy in lowering IOP, thanks to their favorable balance of clinical benefit vs. risk, as well as their cost effectiveness and reversability. However, optometrists must assess the potential for patient non-compliance, inability to instill drops, medication side effects or preservative sensitivities to thwart long term success in medical management alone. This clinical judgment will factor in to the decision on whether—and when—to recommend a MIGS procedure.
Typical medication non-adherence is between 50% and 60%.3 Persistent use of topical therapy may lead to ocular surface disruption and conjunctival inflammation, potentially impacting future surgical options if the condition continues to progress.4 Oral carbonic anhydrase inhibitors are beneficial for patients in need of a significant reduction in IOP, but are typically reserved for emergent situations due to their numerous systemic side effects.
• Laser. Argon or selective laser trabeculoplasty targets pigmented trabecular meshwork cells, administered as an outpatient single-session therapy. It has been shown to lower IOP by 20% to 30%, with efficacy maintained at a success rate of about 75% after 2.5 years.5,6 There are no post-op changes in vision, although patients may note mild discomfort for the first 24 hours and have the potential to develop a low-grade iritis.
Patients may be sent home with a topical NSAID or steroid, or even without drops, based on the doctor’s preference. IOP is monitored seven to 10 days postoperatively.
Pretreatment glaucoma medications are continued for two to three months until the effect of the laser therapy can be fully assessed. Although there are no adverse effects with this intervention, about 20% of patients will demonstrate no change in IOP.7 This procedure may be repeated, although it is thought to be less effective with each additional session.
• Incisional surgery. Trabeculectomy, considered the gold standard of glaucoma surgery, creates an additional source of aqueous drainage, bypassing the trabecular meshwork and ultimately creating a “bleb.” An adjunctive antimetabolite is commonly used by the surgeon to help prevent fibrosis and subsequent failure of the bleb.
Trabeculectomy is typically used for moderate to advanced glaucoma (i.e., one to two quadrants of field loss). This procedure may be advantageous—or perhaps necessary—when trying to achieve a target IOP less than 12mm Hg to 14mm Hg.
One shortcoming of this procedure is its demanding postoperative course. The patient is asked to refrain from bending over, stooping or lifting anything greater than 10 pounds up to three weeks following surgery. These limitations can affect the patient’s ability to work and ultimately function independently. Patients are instructed to use a topical antibiotic drop for the first seven to 10 days, and a topical steroid for the first four to six weeks.
A comanaging optometrist, if involved in the post-op care, would follow the patient very closely in the first few weeks (often one to two times per week for the first two to three weeks after surgery) to monitor the outflow of aqueous, formation of the bleb and to remove sutures. The placement of sutures may affect the patient’s vision and refractive error. Visual recovery and stability often takes two to three months. The postoperative course for these patients is somewhat unpredictable; risks include wound leakage, excessive filtration (leading to hypotony), bleb dysesthesia, bleb failure and the need for subsequent revisions and endophthalmitis.
Glaucoma drainage devices (GDD) aid in the filtration process by shunting aqueous from the anterior chamber to an extraocular reservoir through a tube. This procedure is typically reserved for patients with moderate to advanced glaucoma who have a target IOP less than 12mm Hg to 14mm Hg and may not do well with a trabeculectomy. This procedure is indicated in patients who have uveitis, neovascular glaucoma, aphakia, inadequate conjunctival tissue or a previously failed trabeculectomy.
The postoperative period includes the use of topical corticosteroids for four to six weeks, antibiotics for seven to 10 days and cycloplegics for three to six weeks. Complications of a GDD include over-filtration, tube obstruction, migration or erosion, motility disturbances and endophthalmitis.
MIGS in the Middle
Due to the arduous nature of traditional glaucoma surgery, the search continues for newer procedures that may suffice as a safer alternative to trabeculectomy for patients with mild to moderate glaucoma. The minimally-invasive glaucoma surgeries are a subset of newly developed procedures that offer a faster recovery, lower complication rates, minimal impact on refractive error and preservation of the conjunctival tissue, should filtering surgery be warranted in the future. But, their IOP lowering effect is less robust than incisional surgery as well. These procedures are optimal for patients with a target IOP in the mid/high teens to low 20s, with better IOP control seen when the target IOP is at the higher end of that range.8
Given their comparatively modest benefit in IOP reduction, MIGS procedures are typically combined with cataract surgery to spare the patient another surgical procedure that may otherwise be difficult to justify on IOP modification alone. Indeed, a recent retrospective analysis of the Ocular Hypertension Treatment Study found that cataract surgery itself confers about a 16.5% reduction in IOP.9 The MIGS procedures can be seen as additive to that effort rather than a primary surgery in some cases.
Currently, the Trabectome (NeoMedix) and iStent (Glaukos) are two FDA-approved MIGS devices that modify the trabecular meshwork, either by removing (Trabectome) or bypassing (iStent) the affected tissue, thought to be the major source of outflow resistance.
When a MIGS procedure is being performed in conjunction with cataract surgery, surgeon preference based on individual patient characteristics dictates which procedure will be performed first. While it is often recommended to perform the glaucoma procedure first, the surgeon may elect to begin with cataract extraction on a patient with a shallow anterior chamber or short axial length. Removing the cataract first potentially allows the surgeon improved visualization of the trabecular meshwork and ease the process of the glaucoma procedure.
Prior to these surgical procedures, thorough gonioscopy should be performed to rule out peripheral anterior synechia, neovascularization, angle closure or any other abnormality that would prohibit visualization of the angle or alteration of the trabecular meshwork during the surgery.
• Trabectome. In this procedure, a 19.5-gauge instrument is inserted temporally through a clear corneal incision and directed towards the nasal anterior chamber. Using a surgical gonio lens, the footplate of the device is inserted though the trabecular meshwork into Schlemm’s canal. This procedure ablates 60° to 120° of trabecular meshwork and the inner wall of Schlemm’s canal. The clear corneal incision is then closed with a suture.
Following the procedure, patients are maintained on a topical antibiotic for one week and a topical corticosteroid for three to four weeks. They continue their preoperative glaucoma medications for the first four to six weeks and are instructed to use a topical parasympathomimetic agent (e.g., pilocarpine 1-2% QID or isopto-carbachol 1.5% BID) for the first six to eight weeks. These patients are typically seen for follow-up in one day, five to seven days, two to three weeks and two to three months after the surgery. The most common complications of the Trabectome include early IOP spikes and hyphema.10
The use of a parasympathomimetic drop is to help open the trabecular meshwork, prevent fibrosis and ultimately increase aqueous outflow after the Trabectome procedure. Due to the potential retinal side effects associated with this therapy, a thorough fundus examination is indicated prior to the procedure. Referral to a retina specialist for clearance may need to be considered in patients with high myopia, lattice degeneration or a history of retinal breaks.
Patients may complain of side effects associated with the subsequent miosis of the parasympathomimetic, most commonly a head or brow ache. When comanaging the post-op care, you can alleviate this by gradually increasing the strength of the medication or switching to a different drug in the same class.
For instance, if a patient is having difficulty with pilocarpine 2%, it may be worthwhile to drop down to pilocarpine 0.5% to 1% for the first week and then gradually increasing back to 2% once the patient has acclimated. Another alternative may be to switch from pilocarpine to isopto-carbachol. It is important to educate patients on the importance of this medication on the long term outcome of this surgery, as this often improves compliance with this drop.
• iStent. This device is a 1mm heparin-coated stent inserted with an applicator through a clear corneal incision. Also using a surgical gonioscopy lens, the device is implanted through the nasal aspect of the trabecular meshwork into Schlemm’s canal after being released from the applicator. Recent studies have demonstrated the potential benefit of implanting more than one stent for an additional degree of IOP reduction.11 The iStent can be difficult for surgeons to place, especially in restless patients or those with peripheral anterior synechia or corneal edema. The clear corneal incision is then closed with a suture.
Following the procedure, patients are placed on a topical antibiotic for one week and topical corticosteroid for three to four weeks, and continue their preoperative glaucoma medications for the first three to six weeks. They are typically seen for follow up in one day, seven days, three weeks and two to three months after the surgery. The most common complications of the iStent include elevated IOP and stent obstruction or malposition.12
• Express mini-shunt. While Alcon’s device is sometimes classified within the MIGS category, its similarities to incisional procedures (e.g., bleb formation, postoperative course, potential complications and greater IOP-lowering effect) make it more akin to trabeculectomy. Indeed, one study found better IOP control among Express patients than those who had undergone conventional trabeculectomy.13
The Express is a non-valved stainless steel shunt inserted under a conjunctival flap to shunt aqueous from the anterior chamber towards a subconjunctival reservoir, in a manner very similar to a traditional partial-thickness filter. In practice, the Express mini-shunt is often used in conjunction with a trabeculectomy. The benefit of this device stems from its potential ability to better regulate the outflow of aqueous, presumably decreasing the risk of hypotony and hypotony-related complications that are experienced with a trabeculectomy.
Managing MIGS Post-op
It is important to perform gonioscopy within the first few weeks following these surgeries, as it is the best way to visualize the surgical result. Following the Trabectome procedure, note the clock hours of trabecular meshwork that were removed and monitor for alterations, such as fibrosis of the tissue. While visualization of the iStent may be possible at the slit lamp, a gonioscopic view is required to monitor the stent for potential obstruction or malpositioning.
Fluctuations in IOP are often noted after these procedures. In the first few days following surgery, increased IOP may be related to retained viscoelastic or red blood cells in the anterior chamber, which can clog the trabecular meshwork and cause an obstruction of aqueous outflow. Increased IOP after iStent implantation may be caused by obstruction or malpositioning of the stent. IOP spikes following the surgery should be addressed accordingly through observation, the addition of topical or oral therapy, paracentesis or repositioning/replacement of the iStent by the surgeon, depending on the severity of individual’s glaucoma and the magnitude of the IOP spike.
An IOP response to a steroid should be considered if the IOP elevates after three to six weeks of prolonged steroid use. At this point, tapering off the steroid would be the most effective way to stabilize the IOP.
A hyphema during these procedures is common and usually caused by reflux from Schlemm’s canal. The Trabectome affects a larger area of trabecular meshwork and typically creates a larger blood reflux than the iStent. This most commonly resolves within the first week.
As in cataract surgery, vision should stabilize in the first one to two weeks following these procedures. Patients with a hyphema should be educated that the vision may have more drastic fluctuations until the blood clears, especially after bending over or changing head positions. Patients who are using a miotic should be educated on the effects of the drug and subsequent miosis on the visual system.
A single suture is often used to close the wound for a larger clear corneal incision, complex cataract surgery with phaco, and with some of the MIGS procedures. They are typically left in place long term and should not disrupt the ocular surface or patient comfort. It is critical to stain sutures with sodium fluorescein to examine for breaks and to monitor for exposure. Patients may report pain or a foreign body sensation if a suture has broken and become exposed. Due to the risk of infection by an exposed suture, a topical antibiotic should be used until the surgeon can remove it.
The ultimate goal of treatment should be to achieve the target IOP with the least amount of risk and impact to the patient. With the arrival of the MIGS options, safe and effective surgical alternatives are now available in early to moderate glaucoma. These procedures potentially open the door to earlier prevention of glaucoma-related vision loss. Current research is being directed at improving their IOP reduction without a commensurate increase in risk profile. Investigational devices (e.g., CyPass microstent, Hydrus II, SolX Gold shunt) as well as next-generation versions of the currently approved devices give optometrists and their patients reasons to remain optimistic about the burgeoning field of newer, safer surgeries.
Dr. Young is in practice at Chicago Glaucoma Consultants in Glenview, Ill. and is an assistant professor, adjunct faculty at the Illinois College of Optometry.
All intraoperative images courtesy of Derek Cunningham, OD, and Walter Whitley, OD. Narrated videos of these surgical procedures can be found online in the Surgical Minute archive at www.revoptom.com/multimedia.
1. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: Results from The Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002 Oct;120(10):1268-79.
2. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000 Oct;130(4):429-40.
3. Rees G1, Chong XL, Cheung CY. Beliefs and Adherence to Glaucoma Treatment: A Comparison of Patients From Diverse Cultures. J Glaucoma. 2013 Jan 31.
4. Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106(3):556-63.
5. Damji KF, Shah KC, Rock WJ, et al. Selective laser trabeculoplasty v argon laser trabeculoplasty: a prospective randomized clinical trial. Br J Ophthalmolol. 1999;83:718-722.
6. Melamed S, Ben Simon GJ, Levkovitch-Verbin H. Selective laser trabeculoplasty as primary treatment for open angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol. 2003 Jul;121(7):957-60.
7. McIlraith I, Strasfeld M, Colev G, Hutnik CM. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma. 2006 Apr;15(2):124-30.
8. Ahuja Y1, Ma Khin Pyi S, Malihi M, et al. Clinical results of ab interno trabeculotomy using the trabectome for open-angle glaucoma: the Mayo Clinic series in Rochester, Minnesota. Am J Ophthalmol. 2013;156(5):927-35.
9. Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology 2012 Sept;119(9):1826-31.
10 Mosaed S, Rhee DJ, Filippopoulos T, et al. Trabectome outcomes in adult open-angle glaucoma patients: One-year follow-up. Clin Surg Ophthalmol. 2010;28(8/9):182-6.
11. Hays CL, Gulati V, Fan S, et al. Improvement in Outflow Facility by Two Novel Microinvasive Glaucoma Surgery Implants. Invest Ophthalmol Vis Sci. 2014 Feb 18. pii: iovs.13-13353v1. doi: 10.1167/iovs.13-13353.
12. Craven ER, Katz LJ, Welss MW, et al. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012 Aug;38(8):1339-45.
13. De Jong L, Lafuma A. Aguade AS, Berdeaux G. Five-year extension of a clinical trial comparing the EX-PRESS glaucoma filtration device and trabeculectomy in primary open-angle glaucoma. Clin Ophthalmol. 2011;5:527-33.

