Diabetic macular edema (DME) is a sight-threatening condition and the most common cause of visual loss in patients with diabetes mellitus (DM).1,2 It has a prevalence of 3.8% in diabetic patients over the age of 40, regardless of gender.3 However, elevated hemoglobin A1c and longer duration of DM have a direct association with the prevalence of DME.3 Comorbidities—including hyperlipidemia, hypertension and renal disease—as well as medications such as thiazolidinediones can increase the risk of DME.4,5 By understanding the pathophysiology of DME, early detection and intervention can occur, thereby halting the progression of DME and reducing the risk of permanent vision loss.1,6
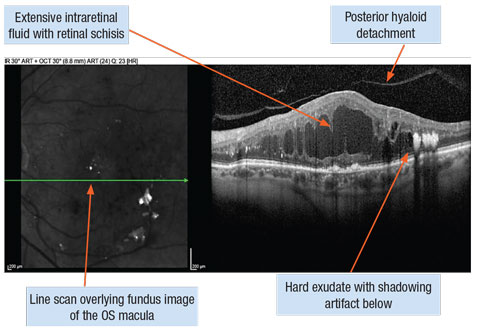 |
| Diffuse diabetic macular edema captured with spectral-domain OCT line scans. Click image to enlarge. |
Pathophysiology
DME is the result of chronic microvascular compromise and can develop by an inflammatory or ischemic mechanism. High plasma glucose levels cause the breakdown of the blood-retinal barrier through the loss of pericytes. This leads to loss of endothelial cell function and release of vascular endothelial growth factor (VEGF).7 This growth factor leads to capillary leakage, causing the accumulation of extracellular fluid in the macula.8,9 VEGF also causes activation of inflammatory molecules and is implicated in neuronal apoptosis and capillary non-perfusion.1
Classifications of DME by Imaging Modality11,13,36,37 | ||||
| OCT | Diffuse | Cystoid: further divided into mild, moderate or severe based on distance of exudates/ thickening from the center of fovea | Posterior hyaloid/ vitreofoveal traction | Serous retinal detachment |
| FA | Focal: localized leakage from microaneurysms/ dilated capillaries | Diffuse: involves entire circumference of fovea | Diffuse cystoid: diffuse leakage, the dye is within the cystic area of macula in late phase of FA |
|
| Subretinal fluid area vitreoretinal interface abnormalities etiology (SAVE) Protocol (OCT/FA) | Focal or multifocal: definable leakage source in FA, edema in OCT | Non-focal: no definable leakage source in FA, edema in OCT | Macular or peripheral ischemia: defined as capillary non-perfusion in FA | Atrophic edema: retinal cystoid degeneration w/o Müller cells or disruption in horizontal layer centrally |
Classification
DME is defined as retinal thickening or hard exudates at least one disc diameter to the center of the macula.11,12 Clinically significant macular edema (CSME), introduced by the Early Treatment Diabetic Retinopathy Study (ETDRS), is defined as DME meeting at least one of three criteria: thickening at or within 500µm of foveal center, hard exudates within 500µm of foveal center with adjacent thickening, or at least one disk diameter of thickening with part of it located within one disc diameter of foveal center.11-13 Treatment is typically required when DME meets the criteria based on ETDRS evidence.12
Evaluation Techniques and Imaging Modalities
Early detection of DME can lead to prompt treatment and reduced risk of permanent vision loss. It can also provide information on how the disease progresses and the response to treatment. Some early detection techniques include:
Slit lamp examination with a contact fundus lens. With the advent of advanced imaging devices, clinicians may overlook the opportunity to define DME during a fundus evaluation. Retinal vascular clues such as bending of the vessels, proximity of hemorrhage/microaneurysms to the macula or presence of hard exudate can alert an astute clinician to the presence of DME. Using a contact fundus lens to evaluate retinal thickness (RT) and elevation secondary to intraretinal or subretinal fluid is also a viable option. Whether the DME is diffuse or focal will determine how easily it is identified on clinical exam.
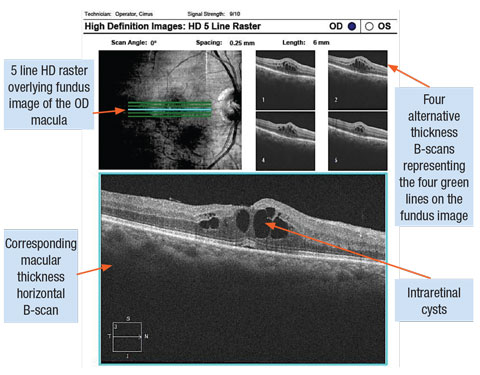 |
| Cystoid diabetic macula edema captured with SD-OCT line scans. Click image to enlarge. |
Conventional color fundus photography. Fundus photos are still useful for monitoring both progression of DR and the response to treatment.13 Fundus photographs are particularly useful with recent advancements in telemedicine, specifically for high risk patients in rural areas to aid in early diagnosis and intervention.13
Fluorescein angiography with widefield functionality. Fluorescein angiography (FA) historically has been considered the gold standard in the diagnosis of DME, as it can illustrate capillary non-perfusion and leakage in the retina.9 Recent advances with widefield FA improve views of the posterior pole and the peripheral fundus, aiding in accurate diagnosis of early ischemia and proliferative diabetic retinopathy. This is vital, as recent studies have shown that peripheral ischemia may be related to the presence and significance of DME.13
Clinicians should be aware that adverse events can occur during FA testing, such as: allergic anaphylaxis, local tissue necrosis, nausea, vomiting, GI distress, cardiopulmonary reactions, headaches, convulsions and thrombophlebitis at the injection site. Clinicians should also be cautious when evaluating pregnant or nursing mothers. The invasiveness of this procedure and the development of OCT-angiography (OCT-A) will reduce the use of FA in the diagnosis and management of DME.9 However, because widefield imaging is currently a limitation for OCT-A, FA remains a valuable diagnostic option for evaluating DME.
Spectral domain (SD) and swept source (SS) OCT. OCT has quickly become the new gold standard for monitoring DME.14 It can quantify volume and thickness to evaluate resolution over time with treatment and can be used to image hard exudate and intraretinal blood within the retinal layers. OCT can define vitreomacular traction and provide an accurate evaluation on the anatomical integrity of the retina, the inner/outer segment junction line and the external limiting membrane.14 Current algorithms can segment out particular retinal layers for individual evaluation, and macular change analysis functions are ideal for looking at thickness differences over time. SS-OCT advancements have provided better visualization of the choroid and choroidal scleral interface. Studies show that subfoveal choroidal thickness is reduced in patients with DME.15 In the future, this could provide a better understanding of the disease process and may lead to possible alternative treatment sites. SS-OCT can also be beneficial in evaluating the vitreoretina interface in patients with diabetes. Recent studies demonstrate adhesion between the posterior hyaloid and the retina in patients with DME not observed with prior technology.13
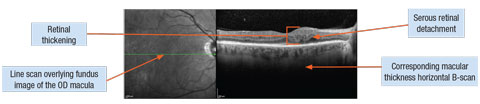 |
| DME associated with serous retinal detachment. Click image to enlarge. |
OCT-angiography. OCT-A is a newer imaging modality that can identify the depth of the retina and choroid. It can detect capillary dilation or truncation, increased foveal avascular zones and capillary dropout or non-perfusion in the retina. This quick, non-invasive, in-office test can define the inner and outer capillary plexus. The choroidal vasculature is also easily identifiable, which has been a disadvantage of FA and why indocyanine green angiography (ICG) is preferred to define choroidal disease. While FA and ICG demonstrate pathology through the presence of leakage and pooling of dye from vessels, OCT-A shows retinal vasculature anomalies and corresponding anatomical changes to the retina associated with DME not obscured by dye leakage.16 Studies show OCT-A can identify the location of microaneurysms adjacent to retinal fluid.16
One major limitation of current OCT-A technology is a smaller scan area compared with widefield FA and fundus photography. Also, projection artifacts from inner retinal layers can be projected to deeper layers of the scan, which can make interpretation and delineation of lesions difficult. This technology is also very sensitive to movement and blink artifacts.14
Management and Referral
Once macular edema is detected, determining the exact etiology of the ocular presentation is key. Differential diagnoses of DME may include pseudophakic cystoid macular edema, a central or branch retinal vein occlusion with macular edema, uveitic macular edema, or macular edema associated with an epiretinal membrane or vitreomacular traction. Once clinicians determine the edema is of diabetic origin, they can refer the patient for treatment or monitor based on current ophthalmological and optometric practice guidelines.
The preferred practice patterns published by the American Academy of Ophthalmology list ETDRS protocol for defining CSME as a threshold to determine timing and necessity of treatment due to the risk of moderate vision loss as defined as a doubling of the visual angle.17 It is also imperative to determine if the DME is involving the center of the macula, which studies show has a 10 times greater risk of moderate vision loss at one year compared with eyes not involving the center of the macula. In cases where the patient may refuse or postpone treatment, three to four month evaluations are recommended to monitor for progression of clinical findings and effects on visual acuity.17 The evidence-based clinical practice guidelines published by the American Optometric Association (AOA) suggest if DME is present but does not meet CSME criteria, clinicians should monitor within two to four months.18
The AOA clinical practice guidelines also indicate that the frequency of eye examinations should be determined based upon the type of diabetes, duration of the disease, age and the predicted ability of the patients to adhere to their treatment plans. Other comorbidities and ocular findings must also be considered in determining referral and follow-up assessments.18 In women who are diabetic, care must be taken to monitor these patients closely due to the risk of progression of diabetic retinopathy and diabetic macular edema during pregnancy.19
As with any guidelines, clinicians must not solely depend upon them to formulate treatment plans. Established relationships with retinal specialists will continue to be the cornerstone in making timely referrals and determining follow-up criteria based on the specific treatment modality and each specialist’s comanagement criteria.
DME Treatment Effects, Dosing and Cost2,41-45 | |||
|
| Average Increased BCVA | Dosing | ~Cost per/ dosage |
| Anti-VEGF intravitreal injections | |||
| Lucentis | ~12-15 letters, ~35-45% had >15 letters @ 2 yrs | Monthly (0.3 mg) | ~$1200 |
| Avastin | ~9 letters @ 2yrs, 32% gained 15 letters @ 2 yrs | Monthly (1.25 mg) | ~$50 |
| Eylea | 10-12 letters, ~33% >15 letters @ 12 months | Bi-monthly (2mg) after five initial doses | ~$1850 |
| Corticosteroids | |||
| Iluvien | 7-8 letters @ 3yrs, ~29% >15 letters @ 3 yrs | One implant, ~3 yrs | ~$8000 |
| Ozurdex | ~8 letters @ 3 yrs, ~22% >15 letters at 3 yrs | One implant, ~6 months | ~$2000 |
Treatment Options
The first step in treatment is to counsel patients on lifestyle modifications such as diet and the systemic control of comorbidities such as blood pressure and cholesterol with tight control of glucose levels.20 The American Diabetic Association currently suggests hemoglobin A1c levels less than 7% for non-pregnant adults.21 The Diabetes Control and Complications Trial in the 1980s demonstrated a 23% reduction in macular edema with intensive blood sugar control.22
Currently, there are four evidence-based therapies for DME: focal and grid laser, intravitreal anti-VEGF injection, intravitreal steroid injection and implant and surgical intervention.23
Laser photocoagulation. In 1971, the Diabetic Retinopathy Study (DRS) determined that when treating eyes with DME, performing focal photocoagulation prior to panretinal treatment reduces risk of progression of DME.7 Also, when patients undergo panretinal laser, divided treatment sessions with lower intensity burns may aid in reducing visual loss.22
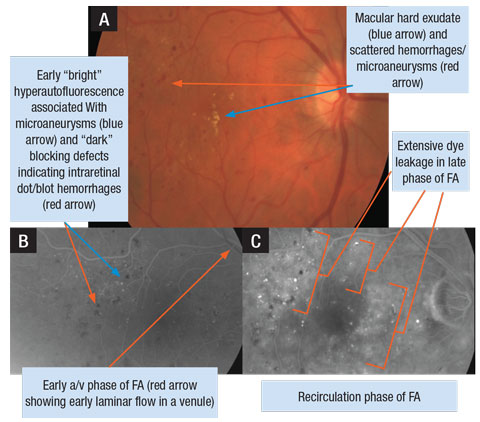 |
| A. Retinal photograph of a patient’s right eye with DME. B. FA photo of early arteriovenous (a/v) phase OD. C. FA photo of later phase (recirculation) demonstrating extensive leakage of dye in the macula OD. Click image to enlarge. |
In the 1980s, ETDRS showed that treating macular edema using macular laser photocoagulation reduced the risk of moderate vision loss, defined by doubling of the visual angle, by 50%, over three years.22 However, patients with entering visual acuity worse than 20/40 had a lesser likelihood of visual improvement after laser treatment.6
Newer therapies such as micropulse laser and laser guided by FA or OCT have been evaluated to reduce damage to the retina during treatment with greater precision and less energy on the retina.24
Intravitreal anti-VEGF injection. Anti-VEGF medications have revolutionized DME treatment. The first anti-VEGF medication FDA approved for the treatment of macular edema was intravitreal Lucentis (ranibizumab, Genentech). Studies show an improvement in best-corrected visual acuity (BCVA) vs. laser, sham and combination therapies.25 Many retinal specialists use off-label Avastin (bevacizumab, Genentech) to treat DME, mostly for financial reasons. Avastin, FDA approved for the treatment of several forms of cancer, acts by a similar mechanism as Lucentis, and studies show it has a similar response, at a fraction of the cost.26 The perceived benefit with Eylea (aflibercept, Regeneron), the latest drug FDA approved for the treatment of DME, is the increased binding of multiple VEGF domains, which would decrease the frequency of intravitreal injections. The DA VINCI study demonstrated that all Eylea groups showed improved BCVA and decreased central RT compared with laser. Other trials showed that five monthly injections, followed by bimonthly injections, had similar increases in BCVA and decreases in central RT, when compared with laser.27
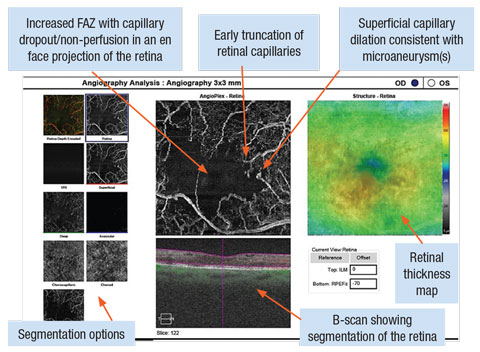 |
| OCT-A with Cirrus OCT Angioplex highlighting a 3x3mm box around the OD macula. The image shows an increased foveal avascular zone with capillary dropout and truncation. Focal dilations of capillary vessels are notated in locations of microaneurysm(s). Click image to enlarge. |
The DRCR.net protocol T study compared the efficacy of intravitreal Eylea, Avastin and Lucentis for DME and found an average increase in BCVA of 13 letters, 10 letters and 11 letters, respectively, with similar safety and side effect profiles.30 The study noted that when initial VA was 20/40 or better no medication had superiority over another; however, when the VA was worse than 20/40 at presentation, Eylea performed better in terms of recovery of vision.28
Intravitreal steroid injection and implants. Because DME may shift from a VEGF-mediated mechanism in the acute phases to an inflammatory mechanism in chronic cases, steroids are a good alternative treatment option for cases of refractory DME, especially in pseudophakic patients.29,30 Current delivery options are peribulbar or intravitreal injection with triamcinolone or fluocinolone acetonide. This mode of treatment suffers from short duration of action, usually one month, and the need for repeated treatments.
An intraocular steroid implant device allows for a slow, sustained release of the drug. The two FDA approved intravitreal implants for DME are Iluvien (fluocinolone acetonide, Alimira Sciences) and Ozurdex (dexamethasone intravitreal implant, Allergan). Clinical trials show Ozurdex can be effective for up to six months, and Iluvien may provide efficacy for up to three years.29,31 Studies show that steroid implants can also work well in combination with other modalities such as laser or surgical intervention.29,32 Most implant candidates have a longstanding history of DME and have likely had macular laser therapy and multiple anti-VEGF injections.33
DME Treatment Potential Side Effects31,36,39,40 | |
| Treatment | Side Effects |
| Focal/grid laser photocoagulation | Risk of foveal burns, subretinal fibrosis, laser scars, central/paracentral scotoma, CNVM, reduced color vision, loss of vision |
| Anti-VEGF
| Endophthalmitis, uveitis, vitreous hemorrhage (VH), retinal tears/detachment, cardiovascular effects from HTN, hypertensive emergencies, arterial thrombo-embolic events, GI perforation |
| Intravitreal steroid | Early cataract formation, elevated IOP, endophthalmitis, VH, retinal tears/detachment |
| Intravitreal NSAID | Endophthalmitis, vitreous hemorrhage, retinal tears/detachment |
| Combination: intravitreal w/laser | See above |
| Pars plana vitrectomy | Endophthalmitis, retinal tears/detachment, vitreous hemorrhage, elevated IOP, cataract |
Intraocular surgical intervention. In cases of DME with vitreoretinal traction, a pars plana vitrectomy (PPV) with or without membrane peel is a viable treatment option.34 In cases of diffuse DME with the presence of subretinal fluid, studies show that PPV was benefical.35 Surgical intervention is not equally effective in all patients with DME, and after vitrectomy there are limitations of increased diffusion and quicker clearance times of drugs by intravitreal injections.34 Alternative therapies such as steroid implants may provide a sustained method of therapy in this group of patients for chronic care after PPV.
Although these therapies have known quantifiable results, they also come with financial, socio-economical and quality of life burdens, such as medication costs, the frequency of the dosing schedule and the decrease in activities of daily living.
Comanagement
The timeline for patient follow up after treatment is based on several factors. Depending on the exact mechanism causing the DME, the response to treatment and the duration of treatment may vary between patients. Other confounding factors such as epiretinal membranes or vitreomacular traction can lead to the need for repeated treatments or the use of multiple treatment modalities, especially in cases of chronic (greater than three-year duration) DME or recalcitrant DME. The location of the DME in the macula (+/- center involvement), the pattern of DME (localized or diffuse) and the retinal perfusion (retinal ischemia) may all alter the treatment course and prognosis. Significant reduction in acuity at initial presentation, other ocular comorbidities and treatment side effects must also be considered for comanagement with your retinal specialist.
International Council of Ophthalmology: Guidelines for Observable Findings of DME38 | ||
| +/- DME | Retinal Findings | Retinal Location |
| (-) DME | (-) thickening or exudates in the posterior pole | X |
| (+) DME | (+) thickening or exudates in the posterior pole | X |
| Mild DME | (+) thickening or exudates in the posterior pole | Outside central macula (diameter 1000µm) |
| Moderate DME | (+) thickening or exudates in the posterior pole | Inside central macula (diameter 1000µm) Not involving the center (fovea) |
| Severe DME | (+) thickening or exudates in the posterior pole | Inside central macula (diameter 1000µm) Involving the center (fovea) |
As the prevalence of diabetes continues to rise in the United States—it increased 382% from 1988 to 2014—the number of individuals with macular edema will consequently rise, increasing the public health burden.3 In 2013, The American Diabetes Association estimated that the United States spends $245 billion every year in health care services and loss of work production in patients afflicted with DM.21
Advances in imaging technology have allowed clinicians to screen diabetes patients and detect DME even before visual compromise. Early detection is crucial in improving patients’ long-term quality of life and reducing the economic burden. The primary eye care providers’ relationship with the referring retinal specialist is integral, as it ensures patients receive the prompt, prudent and individualized care they need. Future research into innovative drug delivery will further change the landscape of DME treatment and management.
Drs. Mazzarella, Cole and Yee are staff optometrists in The Salisbury VA Health Care System, Salisbury, NC.
|
1. Musat O, Cernat C, Labib M, et al. Diabetic Macular Edema. Romanian J Ophthalmol. 2015;59(3):133-6. 2. Calvo P, Abadia B, Ferreras A, et al. Diabetic macular edema: options for adjunct therapy. Drugs. 2015;75(13):1461-9. 3. Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA ophthalmol. 2014;132(11):1334-40. 4. Singh A, Stewart JM. Pathophysiology of diabetic macular edema. International Ophthalmology Clinics. 2009;49(2):1-11. 5. Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med. 2012;172(13):1005-11. 6. Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796-806. 7. May JM. Ascorbic acid repletion: A possible therapy for diabetic macular edema? Free Radical Biology & Medicine. 2016;94:47-54. 8. Lally DR, Shah CP, Heier JS. Vascular endothelial growth factor and diabetic macular edema. Survey of Ophthalmology. April 1, 2016. [Epub ahead of print]. 9. Mookiah MR, Acharya UR, Fujita H, et al. Application of different imaging modalities for diagnosis of diabetic macular edema: A review. Computers in Biology and Medicine. 2015;66:295-315. 10. Melder RJ, Koenig GC, Witwer BP. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nature Medicine. 1996;2(9):992-7. 11. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World Journal of Diabetes. 2013;4(6):290-4. 12. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Survey of Ophthalmology. 2009;54(1):1-32. 13. Tan CS, Chew MC, Lim LW, Sadda SR. Advances in retinal imaging for diabetic retinopathy and diabetic macular edema. Indian J Ophthalmol. 2016;64(1):76-83. 14. ED, Novais EA, Louzada RN, Waheed NK. Contemporary retinal imaging techniques in diabetic retinopathy: a review. Clin Exp Ophthalmol. 2016;44(4):289-99. 15. de Freytas A, Gallego Pinazo R, Cisneros Lanuza A. Subfoveal choroidal thickness in eyes with diabetic macular oedema using swept source optical coherence tomography. Archivos de la Sociedad Espanola de Oftalmologia. 2016;91(5):228-31. 16. de Carlo TE, Chin AT, Joseph T, et al. Distinguishing diabetic macular edema from capillary nonperfusion using optical coherence tomography angiography. Ophthalmic Surgery, Lasers & Imaging Retina. 2016;47(2):108-14. 17. American Academy of Ophthalmology. Diabetic Retinopathy PPP - Updated 2016. Available at www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp-updated-2016. Accessed July 25, 2016. 18. American Optometric Association. Eyecare of the patient with Diabetic Retinopathy: Evidance-Based Clinical Practice Guideline. Available at www.aoa.org/Documents/EBO/EyeCareOfThePatientWithDiabetesMellitus%20CPG3.pdf. Accessed July 25, 2016. 19. Pescosolido N, Campagna O, Barbato A. Diabetic retinopathy and pregnancy. International Ophthalmology. 2014;34(4):989-97. 20. Au A, Singh RP. A multimodal approach to diabetic macular edema. Journal of Diabetes and its Complications. 2016;30(3):545-53. 21. American Diabetes Association. The cost of diabetes. Available at www.diabetes.org/advocacy/news-events/cost-of-diabetes.html. Accessed July 25, 2016. 22. Aiello LM. Perspectives on diabetic retinopathy. Am J Ophthalmol. 2003;136(1):122-35. 23. Apte RS. What is chronic or persistent diabetic macular edema and how should it be treated? JAMA ophthalmol. 2016;134(3):285-6. 24. Luttrull JK, Dorin G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Current Diabetes Reviews. 2012;8(4):274-84. 25. Stewart MW. Anti-VEGF therapy for diabetic macular edema. Current Diabetes Reports. 2014;14(8):510. 26. Ross EL, Hutton DW, Stein JD, et al. Cost-effectiveness of Aflibercept, Bevacizumab, and Ranibizumab for diabetic macular edema treatment: analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol. June 9, 2016. [Epub ahead of print]. 27. Thomas BJ, Shienbaum G, Boyer DS, Flynn HW Jr. Evolving strategies in the management of diabetic macular edema: clinical trials and current management. Can J Ophthalmol. 2013;48(1):22-30. 28. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Eng J Med. 2015;372(13):1193-203. 29. Dugel PU, Bandello F, Loewenstein A. Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin Ophthalmol (Auckland, NZ). 2015;9:1321-35. 30. Al Dhibi HA, Arevalo JF. Clinical trials on corticosteroids for diabetic macular edema. World Journal of Diabetes. 2013;4(6):295-302. 31. Cunha-Vaz J, Ashton P, Iezzi R, et al. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology. 2014;121(10):1892-903. 32. Dang Y, Mu Y, Li L, et al. Comparison of dexamethasone intravitreal implant and intravitreal triamcinolone acetonide for the treatment of pseudophakic cystoid macular edema in diabetic patients. Drug Design, Development and Therapy. 2014;8:1441-9. 33. Bertelmann T, Schulze S. Long-term follow-up of patient with diabetic macular edema receiving fluocinolone acetonide intravitreal implant. Ophthalmology and Therapy. 2015;4(1):51-8. 34. Hernandez-Da Mota SE, Chacon-Lara A, Hernandez-Vazquez E. Use of triamcinolone and bevacizumab in 25G phaco-vitrectomy surgery for the treatment of cataract and diabetic macular edema. Archivos de la Sociedad Espanola de Oftalmologia. 2008;83(5):293-300. 35. Ichiyama Y, Sawada O, Mori T, et al. The effectiveness of vitrectomy for diffuse diabetic macular edema may depend on its preoperative optical coherence tomography pattern. Graefes Arch Clin Exp Ophthalmol. January 18, 2016. [Epub ahead of print]. 36. Mathew C, Yunirakasiwi A, Sanjay S. Updates in the management of diabetic macular edema. Journal of Diabetes Research. 2015;2015:794036. 37. Reznicek L, Bolz M, Garip A, et al. Evaluation of the new “SAVE” protocol in diabetic macular edema over the course of anti-VEGF treatment. Current Eye Research. 2015:1-5. 38. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677-82. 39. Das A, McGuire PG, Monickaraj F. Novel pharmacotherapies in diabetic retinopathy: Current status and what’s in the horizon? Indian J Ophthalmol. 2016;64(1):4-13. 40. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1-32. 41. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmol. 2013;120(10):2013-22. 42. Rajendram R, Fraser-Bell S, Kaines A, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130(8):972-9. 43. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmol. 2014;121(11):2247-54. 58. 44. Campochiaro PA, Brown DM, Pearson A, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmol. 2012;119(10):2125-32. 45. Boyer DS, Yoon YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmol. 2014;121(10):1904-14. |

