Meibomian gland dysfunction (MGD) and tear film insufficiency can severely affect the ocular surface and, if not addressed, can make treatment difficult.1 But any management is dependent upon proper disease identification, which can be tricky with the varied presentations of conditions such as dry eye disease (DED), MGD, allergic conjunctivitis and viral conjunctivitis.
Point-of-care (POC) testing allows diagnostic equipment to aid the ophthalmic exam and to facilitate optimal patient care while reducing patient cost and the optometrist’s time.2 These tests, however, can only help when the clinician understands the nature of these tests and how to use them for best patient care.
POC testing provides quick diagnosis and rapid treatment.3 Nevertheless, they are only adjunctive tests meant to complement a clinical exam that includes patient history, slit lamp evaluation and dilated fundus exam. This article provides a guide on what POC tests are available for patients with ocular surface conditions, when to apply them and how to incorporate them into your standard testing protocol to assure your patients are diagnoses accurately and monitored regularly.
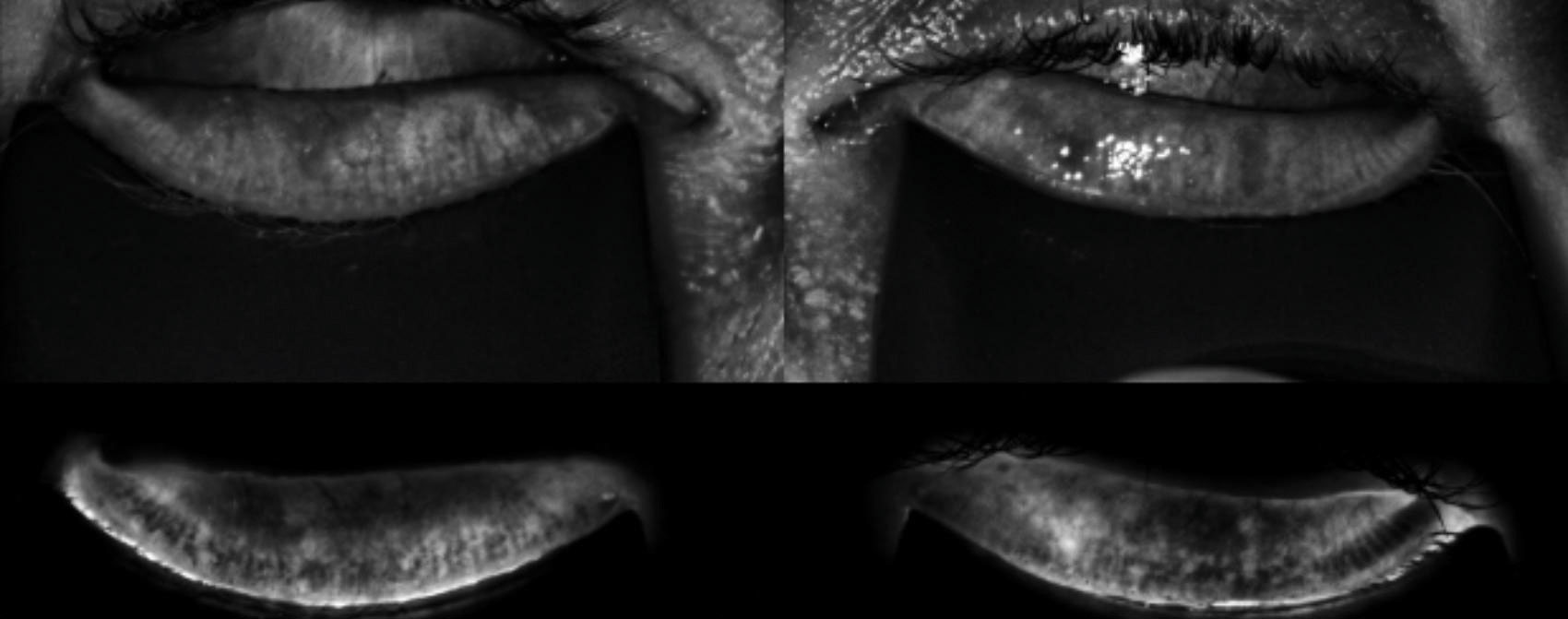 |
| The LipiScan image shows the meibomian glands using near infrared illumination in a quick, noninvasive, in-office test. |
Meibomian Glands
According to the recent publication in the 2017 Tear Film and Ocular Surface Society DEWS II report, dry eye is “a multifactorial disease of the ocular surface characterized by loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”4
Any clinical exam used in conjunction with POC testing must focus on distinguishing what amount of their disease is evaporative in nature—such as MGD—and what amount stems from aqueous deficiency.4 Prior to this recent publication, researchers suggested that these two types of dry eye did not overlap, but that thinking has since been turned on its head.4 However, POC testing can provide the kind of information that can guide an optometrist’s next step. One example is the state of the meibomian glands. MGD can result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation and ocular surface disease. Fluorescein staining, tear break-up time and clinical imaging of the glands will give the practitioner a hint about the root of the patient’s symptoms.
Meibography has become a critical tool in how optometrists diagnose and treat MGD. Tools like these can help establish the patient’s level of gland atrophy and help the clinicians determine whether treatments such as a warm compresses will suffice, or if their disease is too far progressed for that kind of solution.
LipiScan (TearScience) provides a noninvasive image of the glands in 10 seconds. The glands are imaged using near infrared illumination from multiple light sources, minimizing reflection and modifying the light intensity across the surface to compensate for lid thickness variations.5
The LipiView II (TearScience) offers the same high definition imaging along with new functions, including real-time visualization of the lipid layer to evaluate the dynamic response of lipids to blinking, noise canceling technology to measure submicron thickness of the lipid layer and video analysis of blink dynamics. Unlike the LipiScan, the LipiView does not have a screen monitor attached. To display the results on a screen for patients, an extra monitor needs to be attached.
Meibox (Box Medical Solutions) is a portable slit-lamp mounted meibographer compatible with more than 35 slit lamp models. The Meibox offers image processing, which includes dynamic images that enhance the outlines of the meibomian glands. The images are stored on cloud-based software and can be accessed anywhere. The Meibox can be attached to your computer via a USB cord. Similar to LipiScan and LipiView, Meibox is technician friendly, and due to its portability, technicians can bring the Meibox to the patient, reducing chair time.
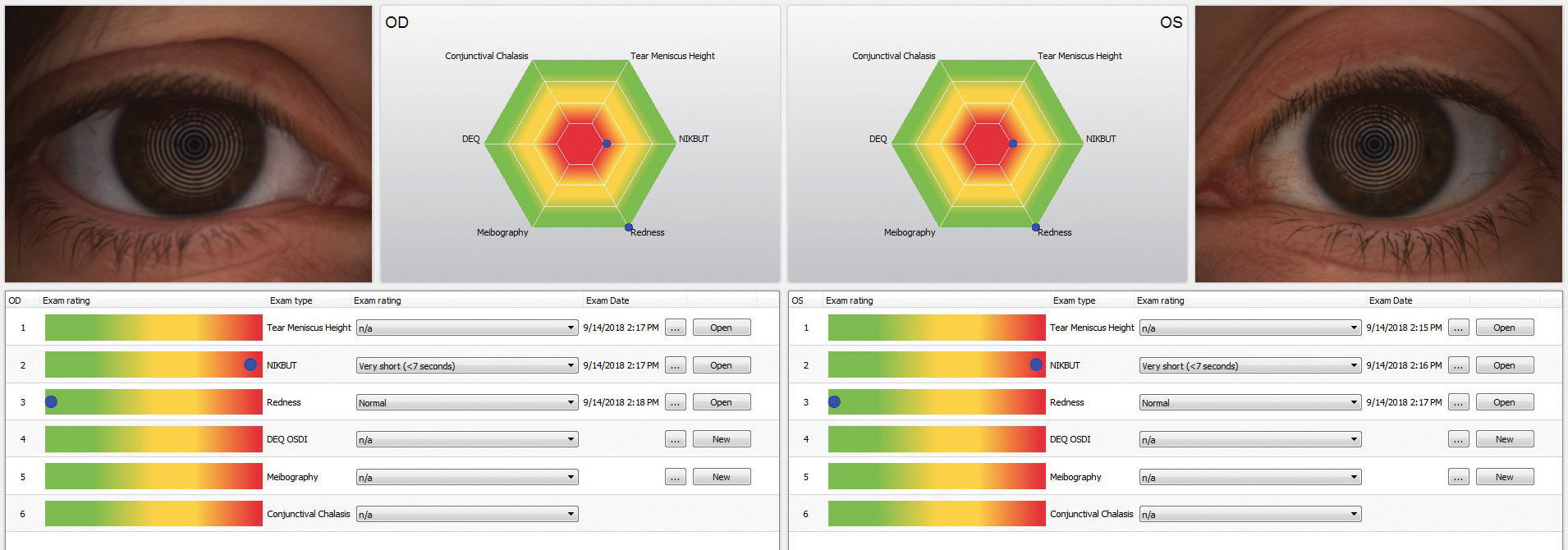
The Keratograph 5M (Oculus) provides information about both the meibomian glands and the tear film as well as advanced placido ring corneal topography, with a built-in keratometer and a color camera included for external imaging.6 Its Meibo-Scan software uses infrared light to image the meibomian glands in high definition. Tear film dynamics are measured with white light, and a quantitative measurement of the tear meniscus height and the non-invasive tear break up time.
Along with the tear meniscus height and non-invasive tear breakup time, a lipid layer interference and particle flow assessment are available. The particle flow assessment measures the tear film particle velocity by tracking the reflective particles in the tear film. The Keratograph uses blue diodes for fluo-images, which can be used for documentation of your slit lamp fluorescein exam. After the Keratograph has collected this data, it offers a final report that offers visual pie graphs, explanations and abbreviations the patient will understand.
 |
 |
| The Keratograph 5M (Oculus) is a multi-functional, noninvasive, in-office device that can provides information about meibomian glands and the tear film. It also offers advanced placido ring corneal topography with a built-in real keratometer with a color camera included for external imaging. |
Osmolarity
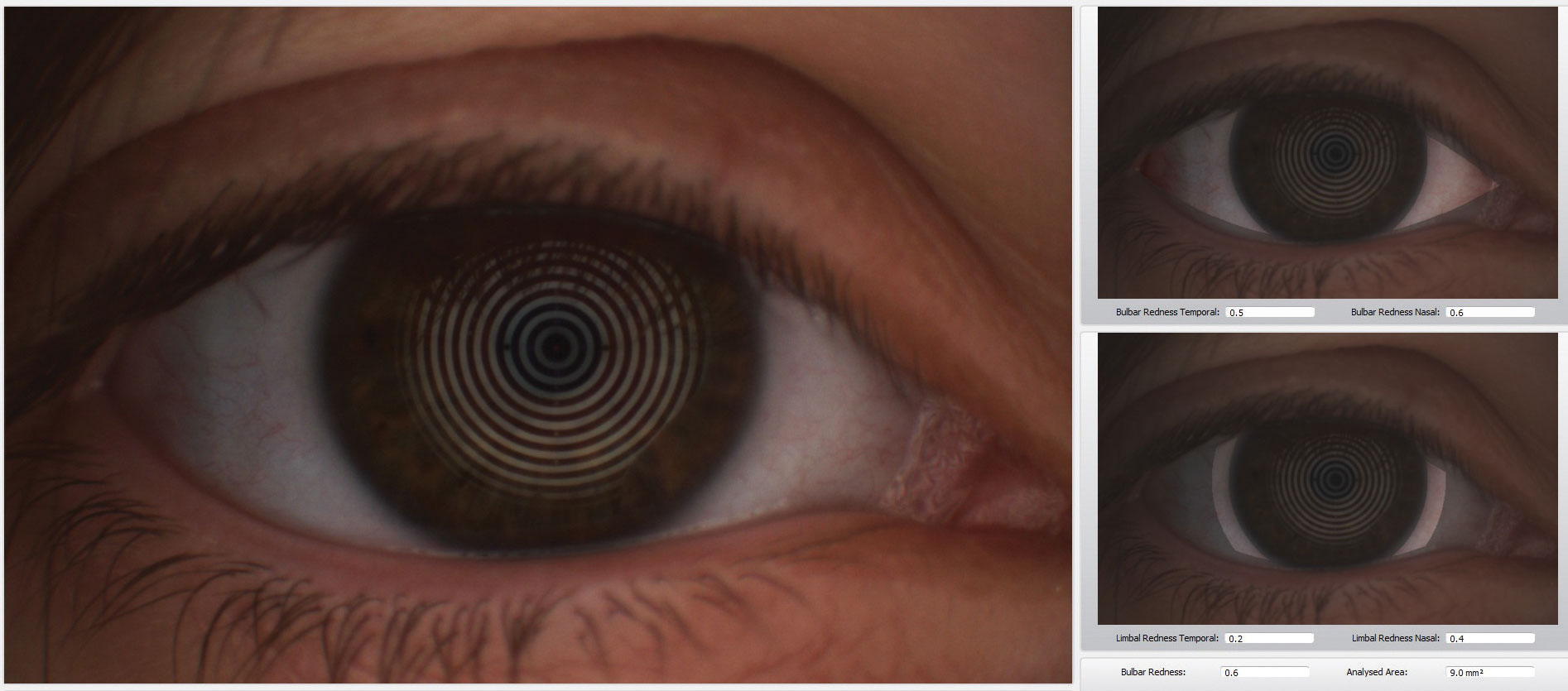
Tear osmolarity is the central pathophysiologic mechanism for all forms of dry eye disease.4 It reduces the ability of mucins to lubricate due to inflammation and cell apoptosis. This ultimately leads to breakdown of homeostatic control and eventually causes tear film instability.7
The Tearlab Tear Osmolarity system has 88% specificity and 75% sensitivity in detecting mild to moderate dry eye disease, and 95% sensitivity in detecting severe dry eye disease, according to the manufacturer.8 The DEWS II section on tear osmolarity reports that new questions arise about the variability of the current measurement technique with TearLab. Various cut-off values have been reported in the literature ranging from 305mOsm/L all the way to 316mOsm/L, as well as a variety of sensitivities, specificities and positive predictive values have been published, which differ from the manufacturer’s data.4
Even though we are still learning and researching the proper way to measure tear osmolarity, TearLab Osmolarity System gives us objective quantitative measurements that allow practitioners to follow appropriate dry eye management in office.
Another option to test tear osmolarity is the I-Pen (I-Med Pharma). That device employs single-use sensors to gather a sample with approximately two to five seconds of contact with the tear-soaked palpebral conjunctiva, rather than a liquid sample as the TearLab system uses. This may be appropriate for patients with severe dry eye as it may be difficult to obtain a tear sample from them. Once a sample is obtained, the I-Pen measures the electrical impedance in the tear-soaked tissues and calculates the osmolarity of the tear film.9
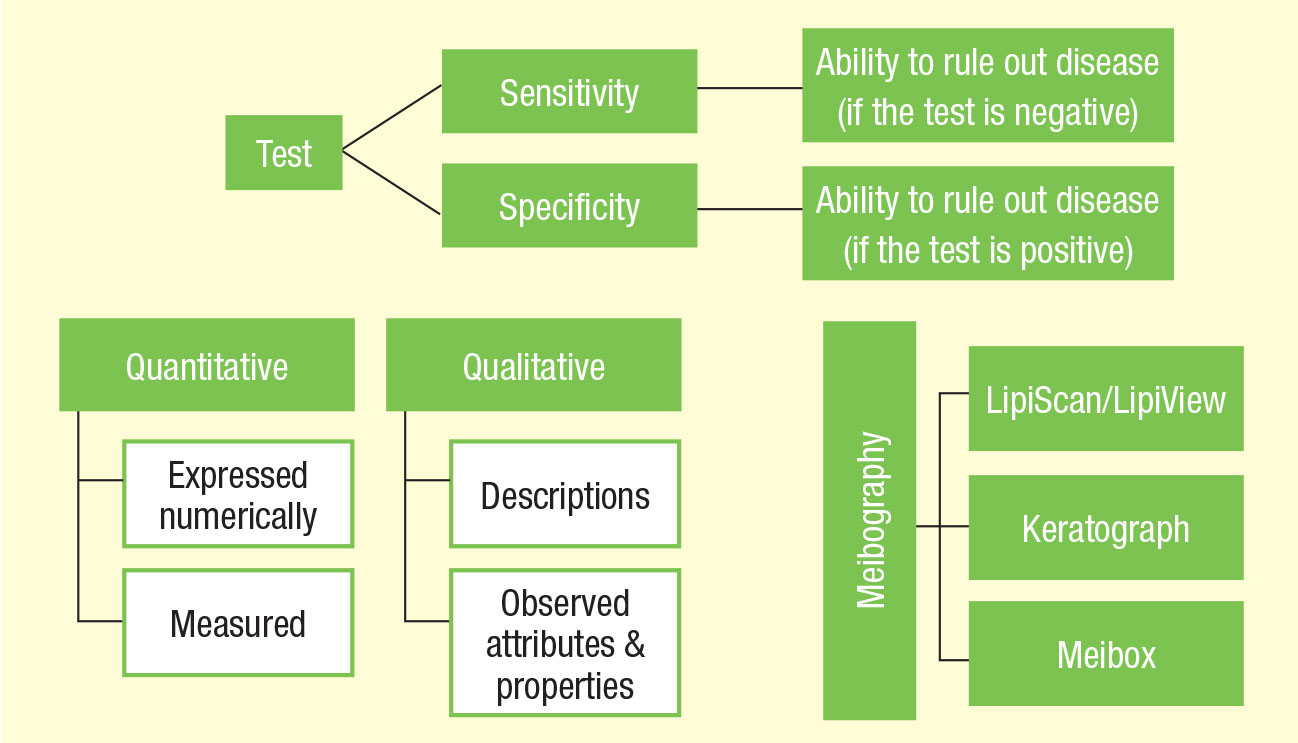 |
| These flowcharts are designed to help guide clinicians in the terminology and use of point-of-care tests. |
MMP-9
Usually, dry eye treatment involves artificial tears and, possibly, punctal plugs. But if the patient has an inflammatory component, practitioners need to think twice about plugs and instead consider anti-inflammatory agents. The MMP-9 Rapid Pathogen Screening InflammaDry test (Quidel) is a non-specific measure of the presence of matrix metalloproteinase-9 (MMP-9), an enzyme that is elevated in dry eye patients.10 MMP-9 testing should be performed before administering ocular anesthetic, topical dyes or Schirmer test. Tear samples are collected from the palpebral conjunctiva with a sample collector fleece. When it starts to glisten, this indicates that the sample fleece is saturated. This is then placed within the test cassette with the addition of buffer solution.
If there is an MMP-9 antibody-antigen interaction on the immunoassay test trip, the result window will read positive with two lines (one blue and one red) within 10 minutes. This is the qualitative result (yes or no). According to the manufacturer, the intensity of the red lines should be directly related to the amount of MMP-9 present.8 The lower detection limit is 40ng/ml, which means that at 40ng/ml, 100% of people will see a positive result. Between 30ng/ml to 40ng/ml, a significant number of patients will be perceived as positive, with more faint positive lines. A negative result will only show a blue line present.8
Studies suggest that MMP-9 presence or absence tell you whether or not a patient will respond to treatment with cyclosporine, doxycycline or steroids, helping reach a more efficient treatment plan with patients.11,12
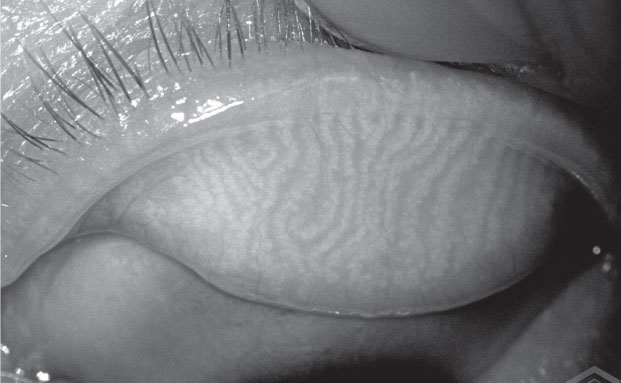 |
| The Meibox produces enhanced images of the meibomian glands that can be stored on cloud-based software for access from any location. |
Viral Infection
Conjunctivitis affects approximately six million Americans annually.13 The common presentation and symptom of “red eye” requires clinicians to include conjunctivitis in their list of differentials, while also making sure to rule out other etiologies, such as dry eye and uveitis.13 Viral conjunctivitis, unlike bacterial conjunctivitis, can present with varied symptoms that coincide with other diseases, even bacterial or allergic conjunctivitis.13 A timeline of symptoms should be noted in the patient history to ensure accurate diagnosis.
A viral etiology can comprise 20% to 70% of infectious conjunctivitis, with adenovirus comprising 60% to 90% of the causes.14 Adenovirus can cause pharyngoconjunctival fever, epidemic keratoconjunctivitis, acute nonspecific follicular conjunctivitis and chronic keratoconjunctivitis.15 Research shows misdiagnosis of conjunctivitis is extremely common amongst clinicians.15 The similarities in signs and symptoms are often to blame for the incorrect diagnoses, leading to inappropriate treatment, prolonging and spreading the disease. It is always important to watch for the hallmark signs of the disease, such as serous discharge, chemosis, pseudomembranes or a follicular reaction.15 Viral conjunctivitis can also be accompanied by preauricular lymphadenopathy, which clinicians can easily test for in-office.
Viral conjunctivitis can take longer to culture for a definitive diagnosis. Laboratory testing for the adenovirus is not commonly used, however, because of the delay in obtaining results; viral cell culture with confirmatory immunofluorescence assay (CC-IFA) need to be evaluated over a 14-day growth period.13 Polymerase chain reaction (PCR) is increasingly used more often because it has higher sensitivity than CC-IFA and can produce results in four hours, but still requires a laboratory to analyze the test sample.14 The difficulty with CC-IFA, PCR and antigen testing, such as enzyme immunoassays, is the laboratory service. Most clinicians either do not have labs within their office or do not have access to labs that can deliver results as quickly as required for accurate diagnosis. Because these tests require multiple steps, a CLIA waiver cannot be obtained for in-office testing.
There is, however, the FDA approved and CLIA waived AdenoPlus (Quidel). The practitioner cost is estimated at $105 per box, with 10 tests per box. The test uses immunoassay technology and delivers results within 10 minutes.13,14 Tear fluid is taken from the inferior palpebral conjunctiva and placed onto a sample collector, which is transferred to a test strip. The strip is dipped into a buffer that allows the antigens to bind to the antibodies, resulting in either a positive or negative result.
Reading the test is also simple because it displays one line for a negative result and two lines for a positive result, a similar reading like the ones found in pregnancy tests. The process is said to be able to identify the 53 serotypes of adenovirus.13
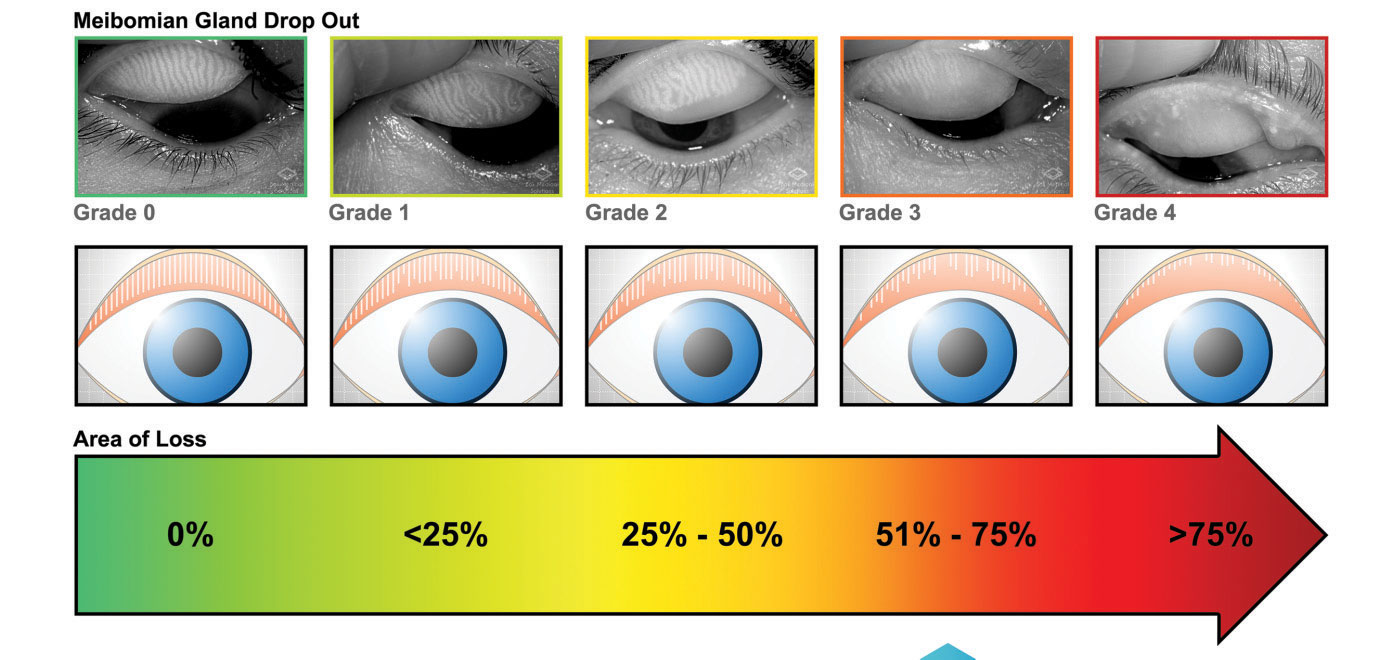 |
| The Meibox device helps physicians determine the grade of gland drop out in MGD. |
Allergic Conjunctivitis
Ocular allergy is an inflammatory disease that affects the ocular surface. It can be divided into four categories: allergic conjunctivitis (including seasonal and perennial), atopic keratoconjunctivitis (AKC), vernal keratoconjunctivitis (VKC) and giant papillary conjunctivitis (GPC).15 The latter three are more severe forms of the disease.
Type 1 hypersensitivity reactions more commonly occur, wherein an offending antigen triggers the allergy cascade. The early-phase reaction occurs with the release of chemical mediators, including histamine, leukotriene and prostaglandin, into the conjunctiva after degranulation of mast cells.16 This is where mast cell stabilizers, (e.g., Pataday, Zaditor) and antihistamine agents (e.g., Azelastine, Bepreve, Lastacaft) treat the disease process. The late-phase reaction occurs with the presence of eosinophils and type 2 helper cells.16 This is treated with corticosteroids and immunosuppressive agents.
Determining the presence of the chemical mediators and cells present in the conjunctiva is key to appropriately treating the disease. The need to test for specific biomarkers on the ocular surface aids in identifying the target area for treatment and improved patient care.
The IgE-mediated response in the conjunctiva is well documented in allergic conjunctivitis, vernal and atopic keratoconjunctivitis.17 With vernal, there is greater involvement by T-cells, eosinophils and cytokines. Atopic keratoconjunctivitis also has T-cell participation, with more TH1-mediated cells.
Advanced Tear Diagnostics has produced a microassay test called Tear Scan that can specifically test for IgE and lactoferrin.17 The test checks for total IgE antibodies in the tear sample. A 0.5µl tear sample mixed with a diluent is placed on the test strip, and after approximately three minutes, the test results can be evaluated. This is beneficial in obtaining an appropriate diagnosis for a patient’s symptoms, ruling out aqueous dry eye disease as a potential diagnosis, leading to appropriate treatment.
The second portion of the Tear Scan microassay system evaluates lactoferrin, a protein with antibacterial, antiviral, antifungal and antiparasitic properties found in a variety of human fluids, including tears.18 The main lacrimal gland secretes a majority of lactoferrin, but ocular epithelial cells and meibomian glands are also contributors.18 An average lactoferrin amount is approximately 1.42mg/ml. Over time, with age and conditions such as dry eye, keratitis and conjunctivitis, a person’s naturally produced ocular lactoferrin decreases.19 The loss of lactoferrin causes greater susceptibility to infections.19 The Tear Scan microassay for lactoferrin has a similar protocol to the IgE test.17 A 0.5µl sample of tear fluid is mixed with a diluent, shaken and deposited into a well. The microassay then measures the amount of lactoferrin. Any amount lower than 1.4mg/ml is considered abnormal.20
The ability to test for both IgE and lactoferrin in combination allows for better diagnosis of aqueous deficient dry eye excluding or including ocular allergy. Being able to diagnose both conditions allows for a better approach to treatment because now treatment can be initiated for each disease.
The Ocular Allergy Diagnostic System-Doctor’s Rx (Bausch + Lomb) offers another in-office test for allergy testing.21 The Doctor’s Rx is FDA approved and can be billed to insurance. It is a noninvasive, requires no needles and responds to at least 58 allergens, according to B+L.21 The test involves a plastic applicator that is applied to the patient’s forearm, but it does not prick or draw blood. The test takes three minutes to perform and provides results within 15 minutes. Testing for allergens, especially those specific to a region, makes it easier to identify an appropriate treatment course and how to remove the offending agent. This will only increase improvement in patient care and satisfaction.
Forego The Lab
As new technologies are developed, practitioners can develop novel ways to treat patients, monitor their compliance and track their outcome. Each of these tests can be performed by the OD—or a properly trained technician, adding efficiency to a busy daily schedule. With these tests, optometrists are no longer limited in our ability to diagnose and monitor by the time constrains and expense of lab testing for our patients’ many ocular surface issues.
Dr. Sherman is an instructor in Optometric Science (in Ophthalmology) at Columbia University Medical Center, NY. She specializes in complex and medically necessary contact lens fittings and ocular disease.
Dr. Shuja is an optometrist at New York-Presbyterian Hospital.
1. Scott C, Catania L, Larkin K. Care of the patient with ocular surface disorders. American Optometric Association. www.aoa.org/documents/optometrists/CPG-10.pdf. December 2010. Accessed October 23, 2018. 2. Bowling E. How point-of-care diagnostic lab tests help clinical decisions. Optometry Times. www.optometrytimes.com/modern-medicine-cases/how-point-care-diagnostic-lab-tests-help-clinical-decisions. May 9 2016. Accessed October 23, 2018. 3. Yesterday, Today & Tomorrow: NIH Research Timelines. National Institutes of Health, U.S. Department of Health and Human Services. www.report.nih.gov/nihfactsheets. June 30, 2018. Accessed October 31, 2018. 4. Craig J, Nichols K, Akpek E. TFOS DEWS II Report Executive Summary. The Ocular Surface. Ocul Surf. 2017;15(3):276-83. 5. A Versatile Range of MGD Products Suited to Your Practice. TearScience. tearscience.com. Accessed October 31, 2018. 6. Oculus, Inc. Oculus Keratograph 5M—Topography Highlights. www.oculus.de/us/products/topography/keratograph-5m/highlights/. Accessed October 31, 2018. 7. Bowling E. The clinical use of ophthalmic point-of-care diagnostic lab tests. American Academy of Optometry 2014. www.aaopt.org/docs/default-document-library/as-15-outline.pdf?sfvrsn=85b3f99e_0. American Academy of Optometry. November 15, 2014. Accessed October 31, 2018. 8. Foulks G, Lemp M, Berg M, et al. TearLab Osmolarity as a biomarker for disease severity in mild to moderate dry eye disease. American Academy of Ophthalmology PO382, 2009. www.tearlab.com/pdfs/2009%20AAO%20Poster%20Handout%20-%20Reduced.pdf. Accessed October 31, 2018. 9. Hessen M. All about osmolarity. Rev Optom. 2018;155(5):42-8. 10. Sambursky R, Davitt W, Friedberg M, Tauber S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea. 2014;33(8):812–8. 11. Chotikavanich S, de Paiva CS, Li de Q, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:7:3203-9. 12. Gürdal C, Genç I, Saraç O, et al. Topical cyclosporine in thyroid orbitopathy-related dry eye: Clinical findings, conjunctival epithelial apoptosis, and MMP-9 expression. Curr Eye Res. 2010;35:9:771-7. 13. Azari A, Barney N. Conjunctivitis: a systematic review of diagnosis and treatment. JAMA. 2013;310(16);1721-9. 14. Sambursky R, Trattler W, Tauber S, et al. Sensitivity and specificity of the AdenoPlus test for diagnosing adenoviral conjunctivitis. JAMA Ophthalmol. 2013;131:17-22. 15. O’Brien T, Jeng B, McDonald M, Raizman M. Acute conjunctivitis: Truth and misconceptions. Curr Med Res Opin. 2009;25(8):1953-61. 16. Shoji J, Aso H, Inada N. Clinical usefulness of simultaneous measurement of the tear levels of CCL17, CCL24, and IL-16 for the biomarkers of allergic conjunctival disorders. Curr Eye Res. 2017;42(5):677-84. 17. Sanchez-Hernandez, M, Montero J, Rondon C, et.al. Consensus document on allergic conjunctivitis. J Invest Allergol Clin Immunol. 2015;25(2);94-106. 18. Rageh A, Ferrington D, Roehrich H. Lactoferrin expression in human and murine ocular tissue. Curr Eye Res. 2016;41(7): 883-9. 19. Rusciano D, Pezzino S, Oliveri M. Age-related dry eye lactoferrin and lactobionic acid. Ophthal Res. 2018;60:94-9. 20. Bethke W. An objective look at dry eye syndrome. Rev Ophthalmol. 2012;19(12):12-3. 21. Doctor’s Rx Allergy Formula: Ocular Allergy Diagnostic System. Bausch + Lomb. www.bausch.com/ecp/our-products/diagnostics/ocular-allergy-diagnostic-system.Accessed October 31, 2018. |

