A new, emerging class of hypotensive drugs—Rho-kinase inhibitors—is being developed as a possible treatment for patients with ocular hypertension (OHT) and primary open-angle glaucoma (POAG).
Many of these drugs are in different stages of development, and those being studied in clinical trials have largely been shown to be safe and efficacious. Here is a short and digestible overview of this new, exciting class of glaucoma drugs.
Are New Drugs Needed?
Currently, the only way we can treat OHT and POAG is to lower intraocular pressure medically or surgically. Oculohypotensive drugs lower IOP in one of three ways:
- Decreasing production of aqueous humor (beta-blockers, carbonic anhydrase inhibitors and alpha-2 agonists).
- Increasing drainage of aqueous humor through the uveoscleral outflow pathway (prostaglandin analogs).
- Indirectly increasing outflow facility through the trabecular meshwork (pilocarpine).
Bear in mind that the trabecular meshwork (TM) accounts for the bulk (70% to 90%) of total aqueous humor outflow.1,2
Although current ocular hypotensive medications are effective, they also have some clear disadvantages. Aqueous suppression agents may decrease oxygen and nutrient supplies to non-vascularized tissue, like the cornea, lens and TM. Prostaglandins do not significantly improve trabecular outflow, and cholinergic drugs typically have unwanted, local tissue side effects, refractive side effects and the potential for systemic issues.3
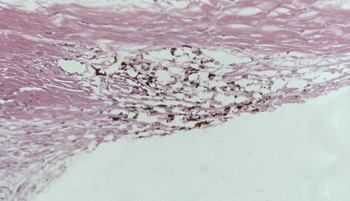
Unlike prostaglandins, which lower intraocular pressure by increasing drainage of aqueous humor through the uveoscleral pathway, Rho-kinase inhibitors improve drainage through the trabecular meshwork (above). Image: Thomas Freddo, OD, PhD
Given these disadvantages, you may wonder: Why haven’t other drugs been developed that target the trabecular pathway? The problem with targeting the TM is that we don’t know the exact mechanism that resists aqueous humor as it traverses the TM and subsequent structures.
(These structures include—in order of the outflow of aqueous humor—the juxtacanalicular connective tissue [JCT], the inner wall of Schlemm’s canal and, lastly, the lumen of Schlemm’s canal. However, most of the resistance to aqueous humor is believed to be generated in the JCT of TM, the inner wall lining of Schlemm’s canal, or both.4-7)
Thus, without having a specific target and clear understanding of the underlying mechanism that regulates resistance to aqueous humor in normal eyes—let alone eyes with glaucoma—investigators have had difficulty developing drugs that increase outflow through the trabecular pathway.
However, recent ocular perfusion studies with drugs that inhibit the Rho-associated kinase (ROCK) pathway in animal models have shown enhanced drainage of aqueous humor through the trabecular pathway, which has led to exploration and development of a promising new class of drugs for treatment of OHT and POAG: Rho-kinase inhibitors.8-11
Rho-kinase Inhibition
Rho-kinase inhibitors (RKIs) work at the cellular level by inhibiting the ROCK signaling pathway. The ROCK signaling pathway promotes cell contractility and adhesion of fibroblast cells (e.g., JCT cells).12-14 Simply put, RKIs induce structural changes to the cytoskeletal framework of fibroblasts that make them more flexible.
Inhibition of the ROCK pathway not only has great promise for treating glaucoma, but also has therapeutic potential for cardiovascular and pulmonary diseases, prostate cancer, neurological disorders and corneal endothelial wound healing.15-21
In the eye, inhibiting the ROCK pathway with RKIs is thought to lower IOP by inducing cellular relaxation and disrupting focal adhesions in the TM and the inner wall endothelial lining of Schlemm’s canal.8
Although the exact mechanism for this increase in outflow facility is unknown, ocular perfusion studies in enucleated cows and monkeys with RKI Y-27632 (a selective inhibitor of specific isoforms for ROCK-I and ROCK-II) have shown significant increases in outflow facility that coincide with an increase in the effective filtration length and separation between the inner wall endothelial cells and underlying JCT (in cows), and between JCT cells and their matrix (in monkeys).9,10 It may be that RKI Y-27632 increases outflow facility by relaxing the cytoskeleton and disrupting the connectivity between inner wall endothelial cells and between JCT cells, which in turn may decrease resistance to aqueous by redistributing outflow patterns through looser regions in the JCT and the inner wall. This hypothesis is supported in similar findings of enucleated pig eyes treated with RKI Y-27632 and monkey eyes with RKI AR-12286.8,22
In human studies, though, the morphological changes in the JCT/inner wall region differed from the animal studies, and therefore warrant further investigation. Specifically, ocular perfusion of RKI Y-27632 in enucleated normal human eyes showed a 134% increase in outflow facility, which correlated with the available area for aqueous humor outflow.23
Topical RKIs Lower IOP
The IOP-lowering effects of topical RKIs have been documented in a number of human studies.
For example, investigators in Japan administered RKI SNJ-1656 to healthy patients at dosages of 0.003%, 0.01%, 0.03%, 0.05% and 0.1% QD or BID, and revealed a dose-dependent drop in IOP at two and four hours post-instillation, with a noted side effect of mild conjunctival hyperemia.24
A study examining the hypotensive effect of topical administration of RKI AR-12286 at dosages of 0.05%, 0.1% and 0.25% in patients with elevated IOP showed clinically significant reductions in mean IOP that were dose-dependent.25 The largest reductions in IOP (28%) were produced with a BID dosage of 0.25% AR-12286, with the only adverse side effect of note being trace to moderate conjunctival hyperemia that lasted four hours or less.
Researchers have shown that combining 0.25% AR-12286 and 0.5% travoprost produces an ocular hypotensive effect that was clinically and statistically greater than travoprost alone, which suggests that a combination therapy of RKI/prostaglandin analog may be a highly effective ocular hypotensive treatment.26
However, this may not be the case with other combinations of RKIs and ocular hypotensive drugs. For example, RKI Y-27632 reduced intraocular penetration of timolol maleate that presumably was due to increased systemic elimination through the conjunctival vasculature.27 Therefore, a multi-drug regimen of RKIs and other ocular hypotensive drugs may require the clinician and patient to be mindful of the order and timing of RKI administration.
Additional Benefits to Ocular Tissue
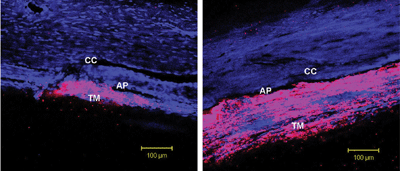
Rho-kinase inhibitors (RKIs) appear to lower IOP by inducing cellular relaxation and disrupting focal adhesions in the trabecular meshwork (TM) and the inner wall endothelial lining of Schlemm’s canal. Confocal microscopic analysis demonstrates the penetration of an RKI in an animal study. In a control eye (left), the tracer dye concentrated in only a small segment of the TM. In an eye treated with RKI (right), the drug was distributed more uniformly throughout the TM.
Reprinted from Scott PA, Lu Z, Liu Y, Gong H. Relationships between increased aqueous outflow facility during washout with the changes in hydrodynamic pattern and morphology in bovine aqueous outflow pathways. Exp Eye Res. 2009 Dec;89(6):942-9, with permission from Elsevier.
Investigators are finding that, in addition to lowering IOP, RKIs have other benefits for the eye:
• Protect human trabecular meshwork cells. Oxidative stress is known to occur in glaucoma and causes upregulation of pro-inflammatory cytokines interleukin (IL)-6 and IL-8, which have been linked to cellular senescence in human trabecular meshwork cells. Cell culture studies have shown that human TM cells treated with RKI Y-27632 exhibit reduced expression of IL-6 and IL-8 mRNA after oxidative stress, which suggests that RKIs may have a protective effect on human TM cells.28
• Improve blood flow to optic nerve. Vasospasm and altered hemodynamics are believed to play a role in certain types of glaucoma, especially normal-tension glaucoma.29-30 Studies in rabbits have provided evidence that systemic or topical application of the RKI fasudil (used to induce vasodilation and improve cerebral blood flow after cerebral vasospasm and stroke) may also suppress impaired blood flow to the optic nerve head.34
• Facilitate corneal endothelium wound healing. Topical RKI Y-27632 has been shown to promote wound healing in primates with partially injured corneal endothelia.21 RKI Y-27632 increased corneal endothelial cell density and restored function in these animals, which suggests RKIs may be a potential therapeutic treatment for certain forms of corneal endothelial cell dysfunction in humans.
• Protect retinal ganglion cells. Glutamate and N-methyl-d-aspartate (NMDA) neurotoxicity have been implicated in retinal ischemia and optic neuropathy, and have been shown to cause degeneration of retinal neuronal cells. In rats with NMDA-induced neurotoxicity, retinal RhoA and ROCK-II protein levels increased and the number of retina ganglion cells decreased; however, intravitreal injection of the RKI fasudil significantly prevented these effects.35
• Decrease fibrosis. One of the main obstacles preventing successful glaucoma surgery is subconjunctival fibrosis. Topical RKI Y-27632 has been shown to decrease subconjunctival scarring after filtration surgery in rabbits.36
Potential Drawbacks
RKIs are potent vasodilators and, when administered topically to the eye, have been shown to induce conjunctival hyperemia and subconjunctival hemorrhages.37 Also, extensive dilation of the conjunctival microvasculature may decrease the effect of concomitantly administered topical drugs by rapidly increasing extraocular clearance from the ocular cavity to the systemic circulation.27
RKIs in the Pipeline
Pharmaceutical companies, such as Senju Pharmaceuticals, Novartis, Kowa, Santen, Aerie, Inspire and others, are exploring ROCK signaling and the effects of RKIs. RKIs are not yet commercially available and many are still in early phases of development. RKIs to be on the lookout for are Y-27632, AR-12286, ATS907, ATS8535, AR-13324 and AMA0076.38-41
With current ocular therapeutics and laser/surgical modalities, the only means by which clinicians and surgeons have the ability to slow or stop the progression of glaucoma is to reduce intraocular pressure.
Recent evidence suggests that other factors (e.g., local autoimmune disorders, oxidative stress, excitotoxicity and mitochondrial dysfunction) may be involved that cause continued disease progression, and IOP reduction alone may be an insufficient treatment in many patients with POAG.
New therapies like Rho-kinase inhibitors could revolutionize the way in which we treat glaucoma. RKIs in early clinical trials appear to be safe and highly effective in lowering IOP, and may even provide neuroprotection to retinal ganglion cells at increased risk in patients with OHT and POAG.
Future investigations may focus on enhanced delivery systems for the drug or prodrug formulations to maximize and localize drug delivery and reduce the risks of unwanted side effects or potential toxicity. Furthermore, proper drug concentration for optimal efficacy, dosing schedule, as well as evaluating potential positive and negative interactions with other therapeutic hypotensive drugs in combination, need to be further evaluated to determine if these agents will be more useful as an adjunct to current treatment strategies or as stand-alone monotherapy.
Dr. Scott is an assistant professor of ophthalmology and visual sciences at the University of Louisville School of Medicine, in Kentucky. He has no financial interest in any of the products or companies mentioned.
1. Townsend BJ, Brubaker RF. Immediate effect of epinephrine on aqueous humor formation in the normal human eye as measured by fluorophotometry. Invest Ophthalmol Vis Sci. 1980 19:256-266.
2. Toris CB, Yablonski ME, Wang YL, Camras CB. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol. 1999 Apr;127(4):407-12.
3. Tian B, Kaufman PL. Comparisons of actin filament disruptors and Rho kinase inhibitors as potential anitglaucoma medications. Expert Rev Ophthalmol. 2012 Apr;7(2):177-187.
4. Grant WM. Further studies on the facility of flow through the trabecular meshwork. AMA Arch Ophthalmol. 1958 Oct;60(4 Part 1):523-33.
5. Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963 Jun;69:783-801.
6. Maepea O, Bill A. The pressures in the episcleral veins, Schlemm’s canal and the trabecular meshwork in monkeys: effects of changes in intraocular pressure. Exp Eye Res. 1989 Oct;49(4):645-63.
7. Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm’s canal in monkeys. Exp Eye Res. 1992 Jun;54(6):879-83.
8. Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho-kinase specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001 Apr;42(5):1029-37.
9. Lu Z, Overby DR, Scott PA, et al. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008 Feb;86(2):271-81.
10. Lu Z, Zhang Y, Freddo TF, Gong H. Similar hydrodynamic and morphological changes in the aqueous humor outflow pathway after washout and Y27632 treatment in monkey eyes. Exp Eye Res. 2011 Oct;93(4):397-404.
11. Tamura M, Nakao H, Yoshizaki H, et al. Development of specific Rho-kinase inhibitors and their clinical application. Biochim Biophys Acta. 2005 Dec 30;1754(1-2):245-52.
12. Hall A. Rho GTPases and actin cytoskeleton. Science. 1998 Jan 23;279(5350):509-14.
13. Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of actin stress fibers and focal adhesions. J Cell Biol. 1996 Jun;133(6):1403-15.
14. Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459-86.
15. Shimokawa H. Rho-kinase as a novel therapeutic target in treatment of cardiovascular diseases. J Cardiovasc Pharmacol. 2002 Mar;39(3):319-27.
16. Mohri M, Shimokawa H, Hirakawa Y, et al. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J Am Coll Cardiol. 2003 Jan 1;41(1):15-9.
17. Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006 Feb 17;98(3):322-34.
18. Fukumoto Y, Matoba T, Ito A, et al. Acute vasodilator effects of a Rho-kinase, fasudil, in patients with severe pulmonary hypertension. Heart. 2005 Mar;91(3):391-2.
19. Somlyo AV, Bradshaw D, Ramos S, et al. Rho-kinase inhibitor retards migration and in vivo dissemination of human prostate cancer cells. Biochem Biophys Res Commun. 2000 Mar 24;269(3):652-9.
20. Mueller B, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005 May;4(5):387-98.
21. Okumura N, Koizumi N, Kau EP, et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013 Apr 3;54(4):2493-502.
22. Wang RF, Serle JB, Kopczynski C. Effect of 0.6% AR-12286 on aqueous humor dynamics in 6 normotensive monkey eyes. Paper presented at Association for Research in Vision and Ophthalmology annual meeting, May 4, 2009; Ft. Lauderdale, FL.
23. Gong H, Yang CY, Liu Y. Effects of rho-kinase inhibitor Y-27632 on aqueous humor outflow facility, hydrodynamics pattern and morphology in human eyes. Paper presented at 20th Biennial Meeting of the International Society for Eye Research, July 21-25, 2012; Berlin.
24. Tanihara H, Inatani M, Honjo M, et al. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch Ophthalmol. 2008 Mar;126(3):309-15.
25. Williams RD, Novack GD, Van Haarlem T, Kopczynski C; AR-12286 Phase 2A Study Group. Ocular hypotensive effect of the Rho kinase inhibitor AR-12286 in patients with glaucoma and hypertension. Am J Ophthalmol. 2011 Nov;152(5):834-41.
26. Levy B, Lewis RA, Kopczynski C, et al. Ocular hypotensive efficacy and safety of a fixed dose combination of AR-12286 (a Rho Kinase Inhibitor) and travoprost. Poster presented at Association for Research in Vision and Ophthalmology annual meeting, May 5, 2013; Seattle.
27. Arnold JJ, Hansen MS, Gorman GS, et al. The effect of rho-associated kinase inhibition on the ocular penetration of timolol maleate. Invest Ophthalmol Vis Sci. 2013 Feb 7;54(2):1118-26.
28. Mochizuki H, Hirata J, Kiuchi Y. Effects of Rho kinase inhibitor on MRNAs associated with glaucoma progression in human trabecular meshwork cells following oxidative stress. Poster presented at Association for Research in Vision and Ophthalmology annual meeting, May 8, 2013; Seattle.
29. Harris A, Sergott RC, Spaeth GL, et al. Color Doppler analysis of ocular vessel blood velocity in normal-tension glaucoma. Am J Ophthalmol. 1994 Nov 15;118(5):642-9.
30. Sako K, Tsuchiya M, Yonemasu Y, Asano T. HA1077, a novel calcium antagonistic antivasospasm drug, increases both cerebral blood flow and glucose metabolism in conscious rats. Eur J Pharmacol. 1991 Dec 10;209(1-2):39-43.
31. Satoh S, Suzuki Y, Harada T, et al. Possible prophylactic potential of HA1077, a Ca2+ channel antagonist and vasodilator, on chronic cerebral vasospasm. Eur J Pharmacol. 1992 Sep 22;220(2-3):243-8.
32. Nagata K, Kondoh Y, Satoh Y, et al. Effects of fasudil hydrochloride on cerebral blood flow in patients with chronic cerebral infarction. Clin Neuropharmacol. 1993 Dec;16(6):501-10.
33. Rikitake Y, Kim HH, Huang Z, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005 Oct;36(10):2251-7.
34. Sugimaya T, Shibata M, Kajiura S, et al. Effects of fasudil, a Rho-associated protein kinase inhibitor, on optic nerve head blood flow in rabbits. Invest Ophthalmol Vis Sci. 2011 Jan 5;52(1):64-9.
35. Kitaoka Y, Kitaoka Y, Kumai T, et al. Involvement of RhoA and possible neuroprotective effect of fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in the rat retina. Brain Res. 2004 Aug 20;1018(1):111-8.
36. Honjo M, Tanihara H, Kameda T, et al. Potential role of Rho-associated protein kinase inhibitor Y27632 in glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2007 Dec;48(12):5549-57.
37. Chen J, Runyan S, Robinson M. Novel ocular antihypertensive compounds in clinical trials. Clin Ophthalmol. 2011 May;5:667-77.
38. Altheos website. Altheos Announces Initiation of Phase 2a Clinical Trial for its Lead Glaucoma Drug Candidate, ATS907. Feb 9, 2012. Available at:
http://altheos.net/9-February-2012-Phase-2a-Clinical-Trial.html. Accessed June 16, 2013.
39. Wirostko BM, Umeno H, Hsu H, Kengatharan M. Safety and efficacy of a novel topical Rho kinase inhibitor ATS8535 in vivo. #5079/A220. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 9, 2012; Ft. Lauderdale, Fla.
40. Wang RF, Serle J, Kopczynski C. Effect of 0.04% AR-13324 on aqueous humor dynamics in normotensive monkey eyes. #1994/D838. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 7, 2012; Ft. Lauderdale, Fla.
41. Hollanders KP, Sijnave D, Van Bergen, et al. The effect of benzalkonium chloride on the intraocular pressure lowering efficacy of a local ROCK-inhibitor (AMA0076). #1974/D818. Poster presented at Association for Research in Vision and Ophthalmology meeting, May 7, 2012; Ft. Lauderdale, Fla.

