24th Annual Glaucoma ReportFollow the links below to read other articles from annual update on glaucoma: MIGS Madness: An Atlas of Options A Guide to Applying IOP-lowering Drops Glaucoma: Lifestyles of the Antioxidant Rich and Famous (Earn 2 CE Credits) |
With today’s advances, glaucoma patients have any number of therapy options, ranging from medical management to highly invasive surgery. But for many patients with open-angle glaucoma (OAG), a simpler option such as laser trabeculoplasty (LTP) can often be the best management strategy. The procedure lowers intraocular pressure (IOP) by treating the trabecular meshwork (TM).
While the mechanism of action is not well understood, its benefit comes from one of three TM changes: mechanical, cellular or biochemical. It is also possible that these effects are synergistic and work together to improve aqueous outflow.1,2 The mechanical theory suggests that the TM absorbs heat from the laser, which alters its structure and stretches its pillars to increase outflow. The cellular theory, on the other hand, postulates that laser therapy alters cellular division to improve the health of the TM cells. Lastly, the biochemical theory proposes that LTP stimulates macrophage-like activity to improve aqueous outflow.
Two different lasers can be used for the procedure, each with slight differences. Here, we take a look at these two options and how you can prepare your patients—and yourself—for the pre- and post-op care.
 |
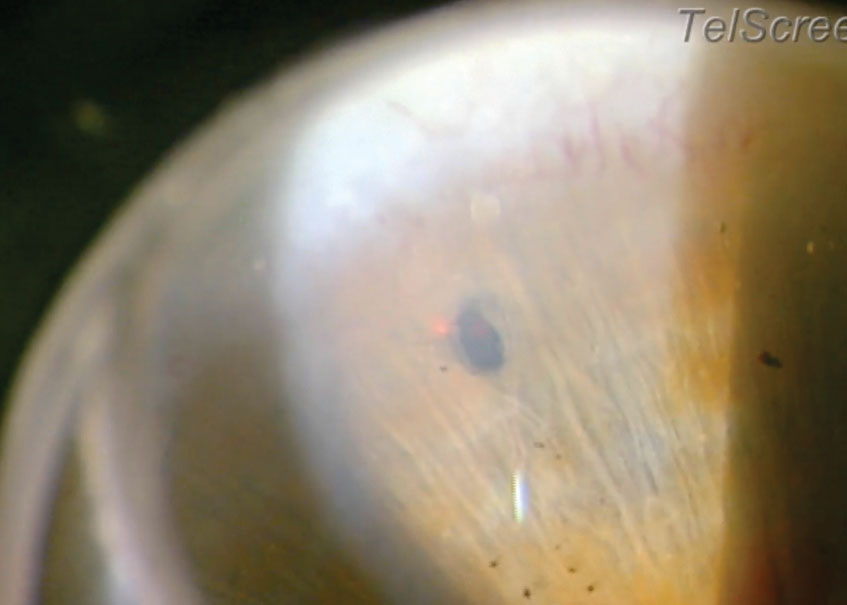
| SLT, using a frequency-doubled short-pulsed Nd:YAG laser, selectively targets pigmented TM cells. Photo: Derek Cunningham, OD. |
ALT vs. SLT
LTP is most commonly administered as either argon laser trabeculoplasty (ALT) or selective laser trabeculoplasty (SLT). While both procedures lower IOP by applying a laser to the TM to increase aqueous outflow, they also have their differences. ALT uses an argon laser to create larger burns and structural alterations to the TM pillars. Although argon laser trabeculoplasty is still the commonly understood term, argon lasers have been replaced with green lasers. SLT uses a frequency-doubled short-pulsed neodymium-doped yttrium aluminum garnet (Nd:YAG) laser that selectively targets pigmented TM cells and minimizes destruction of the surrounding tissue. Efficacy is comparable for both treatments.1,3,4 It is important to note that while efficacy is comparable, ALT is rarely used in clinical practice.
One advantage SLT holds over ALT is repeatability. By minimizing both the amount of energy absorbed and the potential for collateral damage, SLT could theoretically be repeated over time. Although evidence suggests that repeat SLTs are fairly close in effectiveness to the original SLT, current clinical trials are hampered by small sample size and short, 12-month follow-up periods.2,3,5-8 Such short follow-up provides poor evidence for the long-term clinical utility of repeat SLT.
Lasers Go MicroMicropulse laser trabeculoplasty (Iridex), a variation on SLT, targets the TM with pulsed laser treatments to minimize thermal damage. One study of 48 patients found IOP fell by an average of 2.5mm Hg for the SLT group and 3.0mm Hg for the MLT group.
|
Under the Laser
Before the operation, patients are given pilocarpine 1% and Iopidine (apraclonidine 0.5%, Novartis) or Alphagan P (brimonidine 0.1%, Allergan). Pilocarpine, an optional drop some choose to include in the pre-op regimen, helps to contract the ciliary body to open the pillars of the TM and induce miosis to enhance the view of the angle during the procedure. Iopidine or Alphagan P help mitigate possible IOP spikes that may occur during or after the procedure.
Practitioners can then use a gonio lens to visualize the angle before using a fixed 400µm laser spot size for a three nanosecond pulse. Many practitioners start with a lower energy, such as 0.7mJ, and titrate up from there. On average, 45 to 60 laser spots are applied to the TM per 180 degrees. While most physicians treat 360 degrees of the angle one eye at a time, some may prefer to treat less of the angle at a time—even as little as 90 degrees. This is often the case with heavily pigmented TM due to the potential higher energy absorbed by the more pigmented meshwork, especially with ALT. This is less of a concern in SLT, which minimizes structural damage to the TM.3
Post-laser, practitioners should immediately instill Iopidine or Alphagan P and check IOP to ensure no spike exists. Many practitioners send patients home with topical nonsteroidal anti-inflammatory drugs (NSAIDs) to control pain and inflammation. Using topical corticosteroids in these cases is controversial, as many believe that mild inflammation from the laser causes the treatment effect on the TM; however, practitioners may prescribe steroids for these patients to control postoperative inflammation. Recent evidence demonstrates that postoperative anti-inflammatory medications are not necessary after SLT, but using them does not diminish the effectiveness of the procedure.9 Patients should return at a week post-procedure to check for complications such as IOP spike, inflammation, pain and redness. Beyond that, the two-month visit is particularly important for analyzing LTP efficacy, as it may take six to eight weeks for IOP lowering to occur.
All patients undergoing LTP for glaucoma should understand going into treatment that the procedure will not cure their chronic disease or reduce the need for careful follow-up. While many patients are familiar with laser treatments such as laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK), few know about LTP, and managing patient expectations regarding visual outcomes with LTP is crucial.
Patient Selection: A Case ExampleA 75-year-old patient diagnosed with pigmentary glaucoma presented to the office with a 0.65 cup-to-disc ratio, symmetric optic nerves and no focal neuroretinal rim thinning OU. Visual fields show mild visual field loss, and he was pseudophakic following an uncomplicated cataract extraction five years earlier. His untreated IOP has been as high as 26mm Hg OU, with average pachymetry readings. The patient discontinued Cosopt (dorzolamide/timolol, Akorn) due to breathing difficulties and was unable to use Xalatan (latanoprost, Pfizer) due to burning upon instillation. His inability to take topical glaucoma medications was exacerbated by his moderate dry eye syndrome and ocular rosacea. Based on his difficulties with topical medication and the fact that he is pseudophakic, his options are limited to Alphagan P, oral medication, LTP and surgical interventions such as trabeculectomy or tube shunt. Of those options, the patient agreed that LTP has the best risk/benefit profile and chose to pursue SLT. |
Who’s a Candidate, Who’s Not
Ideal LTP patients include those with mild or moderate OAG, especially those with compliance issues such as patients with physical or cognitive disabilities that limit topical medication administration, patients with allergies to topical medication or patients with a general history of noncompliance with topical medication.3,10,11 Other good candidates include patients with pseudoexfoliative glaucoma, pigmentary glaucoma, low-tension glaucoma and ocular hypertension.
Poor candidates for LTP, on the other hand, include patients with narrow angles (due to the need for TM visualization), those with secondary glaucomas (e.g., neovascular, uveitic, iridocorneal endothelial syndrome) and individuals with increased episcleral venous pressure. Those with advanced glaucoma are often not good candidates due to their need for low IOP over long periods of time. Patients with secondary glaucomas tend to need more aggressive forms of treatment such as trabeculectomy or tube shunt.
Young patients are best handled on a case-by-case basis. While they may be good candidates due to the decreased medication burden LTP provides, research shows only 31% maintain treatment effect in five years, suggesting LTP may not be ideal for those who will need long-term treatment efficacy.3
LTP patients with high pre-treatment IOP are likely to require additional medications or surgical intervention. For example, if we expect a typical IOP lowering of 20% from SLT, a patient with a pre-treated IOP of 30mm Hg might need additional lowering if the target IOP was less than 24mm Hg. However, the lower a patient’s pre-treatment IOP, the less successful they are with LTP. In one trial, 32% of patients with IOP of <14mm Hg saw a 1mm Hg increase rather than a decrease. This should be considered for prospective LTP patients with low-tension glaucoma or those with low IOP on existing treatment.12 Despite the difficulty meeting the recommended 30% reduction from the Collaborative Normal Tension Glaucoma Study, there may be other benefits from LTP, such as blunting nocturnal IOP spikes.13
The procedure’s cost may also play a role in patient candidacy. One retrospective trial found LTP was slightly more expensive than medications over a 36-month period ($3,441 for LTP vs. $3,408 for medication). While LTP lowered the cost of pharmaceutical intervention ($807 for LTP patients vs. $1,467 for medication patients), it had a higher medical cost ($2,684 for LTP vs. $1,980 for medication).14
Complications
LTP side effects are transient and self-limited. Most tolerate the procedure without complications, but for the small percentage who do end up with complications, common possibilities including a brief increase in IOP (3mm Hg to 8mm Hg), a mild to moderate anterior chamber reaction, transient conjunctival redness and ocular pain.1-4 Although most postoperative redness and anterior chamber inflammation is self-limited and does not require additional treatment, these side effects can be mitigated by preoperative Iopidine (apraclonidine 0.5%, Novartis), topical NSAIDs and postoperative topical Pred Forte 1% (prednisolone acetate, Allergan) if needed. Although there are case reports of IOP spikes up to 46mm Hg even two weeks post-SLT, most patients will not have complications.14
Additionally, patients undergoing ALT are more likely to develop peripheral anterior synechiae (PAS, 12% to 47%), than SLT patients, who rarely develop PAS.1,4 These synechiae are different than what is seen in angle closure glaucoma or uveitic glaucoma and are not thought to cause long-term complications. PAS in secondary glaucomas are an indication of an active angle closure process, whereas PAS in LTP are localized and non-progressive.
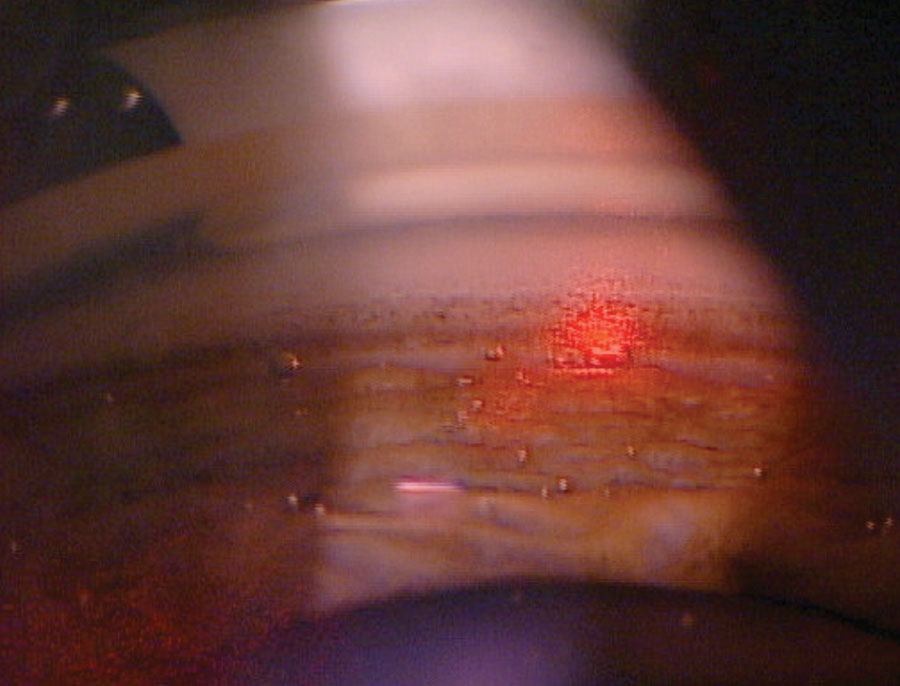 |
| The cavitation or “champagne” bubbles that can be seen throughout the TM during SLT lets the surgeon know the laser is properly interacting with the tissue. Photo: Nate Lighthizer, OD. Click image to enlarge. |
A First-line Therapy?
While laser treatment is quickly growing in popularity—already a first-line option in Europe—it comes with limitations, the most challenging being its short duration of effect. In a short-term LTP study, SLT was as effective as medication at lowering IOP when measured for 12 months. However, research also shows LTP treatment effect diminishes over time, creating a need for a repeat procedure or more medical management.5 Another study shows ALT was successful at lowering IOP by at least 20% in 46% of patients at one year after the procedure, but at five years, this number fell to 13%.3 SLT has more favorable study results, with 58% of patients achieving the same IOP lowering effect as ALT at one year, and 31% maintaining the treatment effect at five years.3 First-line topical medication is typically a prostaglandin analog, such as latanoprost. These typically lower IOP by 35% when taken in the evening. This effect lasted even five years after initiating.16
Lastly, a study that compared LTP with medication management found 40% of LTP patients started a glaucoma medication at 45 days post-procedure, which increased to 80% at the two-year mark. In the medication group, 81% were on two or more classes of glaucoma medication after two years compared with 27% of LTP patients.14
These studies highlight that while ALT and SLT may help lower IOP in the first year or more, practitioners often end up reaching for additional therapies over time.
According to the American Academy of Ophthalmology Preferred Practice Patterns, SLT should be considered as a first-line therapy in select patients, especially for those where noncompliance, cost or side effects of medication are issues.17
Narrow Angles? Consider Another LaserBy Nate Lighthizer, OD While SLT has recently gained traction as a potential first-line option in the treatment of OAG patients, another laser has long been regarded as the treatment of choice in angle closure glaucoma: laser peripheral iridotomy (PI). Laser PI is indicated for the treatment of pupillary block-caused acute angle closure, chronic angle closure, angle-closure glaucoma and narrow angles, among other conditions.1 Recent literature has grouped anatomic narrow angles into three categories:2 Primary angle-closure suspect (PACS). These patients have a narrow angle—classified as failure to see the posterior TM in at least two quadrants on gonioscopy—without any IOP elevation or peripheral anterior synechiae formation. Primary angle-closure (PAC). This is a prior suspect who has now developed either peripheral anterior synechiae or elevated IOP. Primary angle-closure glaucoma (PACG). If primary angle-closure symptoms continue to progress and the patient develops signs of glaucomatous optic neuropathy, the patient is classified as having PACG.
A laser PI is indicated for all cases of PAC or PACG, and many believe it should even be considered for some cases of PACS.2 A laser PI is typically performed with either an Nd:YAG laser or an argon-green laser. Both have their pros and cons, but most eye care providers typically perform the laser PI with an Nd:YAG laser. The most common potential adverse events associated with laser PI, albeit rare, include IOP spike, inflammation, transient blur, hyphema and closure of the PI hole in the weeks following the procedure. Preoperative medications can include an alpha-2 adrenergic agonist such as brimonidine or apraclonidine in-office to lower the risk of IOP spike, as well as pilocarpine to help stretch the iris taut prior to the procedure. Placement of the PI hole has traditionally been along the superior iris at 11 o’clock or 1 o’clock; however, newer evidence suggests a potential benefit for reduced patient dysphotopsias following the procedure when the PI hole is placed temporally.3 The procedure can usually be completed in five to 20 shots, depending on the patient’s iris color with brown irides often requiring more laser shots due to their increased thickness compared to blue irides. Appearance of the pigment plume signifies complete penetration of the iris. Postoperative medications can include brimonidine or apraclonidine in-office as well as the use of a topical steroid for five to seven days post-procedure. A patient with narrow angles and a concurrent visually significant cataract should be evaluated for cataract surgery, as evidence suggests that cataract extraction is superior to laser PI in opening the angles postoperatively.4 In patients without a visually significant cataract, laser PI remains the standard for treating patients with anatomic narrow angles where the posterior TM cannot be visualized in at least two quadrants.
|
Emerging Treatments
Minimally invasive glaucoma surgeries (MIGS) are now FDA-approved to treat mild and moderate primary OAG. MIGS are ab interno procedures, meaning that through the use of existing corneal incisions created during phacoemulsification, the surgeon can minimize conjunctival scarring and trauma—which may occur secondary to conjunctival and sclera incisions necessary for ab externo procedures such as trabeculectomy.
Three major advantages of LTP over MIGS exist: it is an in-office procedure, it avoids incisional surgery and it is repeatable. This leads many practitioners to attempt laser therapy before considering surgical alternatives such as MIGS. However, MIGS do not exclude patients from future LTP applications or ab externo procedures.
Two MIGS procedures employ laser techniques to achieve their IOP-lowering effects:
Excimer laser trabeculostomy (ELT) uses a photoablative probe to vaporize the TM and inner wall of Schlemm’s canal, resulting in a cooling effect and limiting thermal damage. The quartz fiber optic probe, connected to a xenon chloride pulsed excimer laser, is inserted through a clear corneal incision, bypassing the conjunctiva and sclera. With a gonioscopy lens, the surgeon brings the probe into contact with the TM and applies eight to 10 laser spots to create small holes that increase aqueous outflow.18,19 This procedure can be either combined with cataract extraction or performed as a standalone procedure.
Few trials exist to demonstrate the safety and efficacy of ELT. In a two-year prospective study, researchers compared 180 degrees of ELT with 180 degrees of SLT. They found IOP fell from 25±1.9mm Hg at baseline to 17.6±2.2mm Hg for the ELT group and from 23.9±0.9mm Hg to 19.1±1.8mm Hg in the SLT group after 24 months. Glaucoma medications were reduced from 2.27±0.7 to 0.87±0.8 in the ELT group compared with a reduction from 2.20±0.7 to 0.87±0.8 in the SLT group.18
Another clinical trial involved 28 patients who underwent ELT combined with cataract extraction. Researchers split the patients into two groups: those with IOP above 21mm Hg and those with IOP below 21mm Hg at baseline. At 12 months post-procedure, the higher baseline group experienced greater ELT treatment effect than the lower IOP group.18,19 Similar to studies involving LTP, these results suggest patients with a lower baseline IOP are less successful with ELT. None of the 28 patients in the study experienced any serious adverse events, and the most common complication was intraoperative microbleeding, which did not lead to long-term complications.
Because no trials have follow-up periods longer than 24 months, the long-term success and complication rates of ELT are still unknown. While the procedure appears effective with few side effects, many may find adopting this technology difficult because it requires a specialized probe and the use of a surgical suite. When compared with the in-office application of ALT or SLT, in which lasers often have multiple uses, ELT seems less practical.
Endocyclophotocoagulation (ECP), also known as endoscopic photocoagulation, is similar to ELT in that a probe is inserted into a clear corneal incision to spare the conjunctiva, and the procedure can be combined with cataract extraction. However, while other surgical treatment modalities focus on increasing aqueous outflow, the goal of ECP is to lower IOP by reducing aqueous production. ECP is generally used in mild to moderate glaucoma.18
In a two-year trial comparing cataract extraction with ECP to cataract extraction alone for OAG, IOP was reduced from 18.1±3.0mm Hg to 16.0±3.3mm Hg in the ECP/cataract extraction group compared with 18.0±3.0mm Hg to 17.3±3.2mm Hg in the cataract extraction only group at 24 months post-procedure. Medication used dropped from 1.5±0.8 at baseline to 0.4±0.7 at 24 months in the ECP group compared with 2.4±1.0 at baseline to 2.0±1.0 at 24 months for the cataract extraction only group.20
While postoperative IOP reduction was similar between the groups, it required more medications in the cataract extraction alone group. The ECP group did report some side effects, with four patients developing postoperative cystoid macular edema (CME) compared with only one in the cataract extraction alone group. Patients with other CME risk factors such as diabetes mellitus or uveitis may not be good candidates for ECP. Otherwise, complications were uncommon.18
Like ELT, ECP’s limitations include the need for surgical intervention and use of a specialized probe. Unless a surgeon plans to do many of these procedures, purchasing this specialized equipment may be cost prohibitive.
Today, we have more medications than ever at our disposal, traditional surgical treatments have undergone many improvements and less invasive IOP-lowering treatments such as MIGS are available for certain cases. Despite this, LTP still plays an important role for patients with mild to moderate OAG, pseudoexfoliative glaucoma and pigmentary glaucoma. In these cases, LTP can be enough to prevent more invasive intervention.
As every intervention has its pros and cons, LTP included, the future of glaucoma care will involve creative use of all modalities to individualize care.
Dr. DeWilde practices at the Kansas City VA Medical Center and specializes in the diagnosis and management of ocular disease.
1. Reiss GR, Higginbotham EJ, Wilensky JW. Laser trabeculoplasty: a major review. Surv. Ophthal. 1991;35(6):407-28. 2. Samples JR, Singh K, Lin SC, et al. Laser trabeculoplasty for open-angle glaucoma. A report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:2296-302. 3. Barkana Y, Belkin M. Selective laser trabeculoplasty. Surv Ophthalmol. 2007;52(6):634-54. 4. Garg A, Gazzard G. Selective laser trabeculoplasty: past, present, and future. Eye. January 5, 2018. [Epub ahead of print]. 5. Katz LJ, Steinmann WC, Kabir A, et al. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21(7):460-8. 6. Garg A, Gazzard G. Selective laser trabeculoplasty: past, present, and future. Eye (Lond). January 5, 2018. [Epub ahead of print]. 7. Francis BA, Loewen N, Hong B, et al. Repeatability of selective laser trabeculoplasty for open-angle glaucoma. BMC Ophthalmology. 2016 Jul 28;16:128. 8. Hong BK, Winer JC, Martone JF, et al. Repeat selective laser trabeculoplasty. J Glaucoma. 2009;18(3):180-3. 9. DeKeyser M, De Belder M, De Groot V. Randomized prospective study of the use of anti-inflammatory drop after selective laser trabeculoplasty. J Glaucoma. 2017;26(2):e22-e29. 10. Ramulu PY, Parrish RK. Asking the right wuestion in laser trabeculoplasty: “which patient”, not “which laser”? Surv Ophthalmol. 2008;53(6):652-4. 11. Miki A, Kawashima R, Usui S, et al. Treatment outcomes and prognostic factors of selective laser trabeculoplasty for open-angle glaucoma receiving maximum-tolerable medical therapy. J Glaucoma. 2016;25(10):785-9. 12. Pillunat KR, Spoerl E, Elfes G, Pillunat LE. Preoperative intraocular pressure as a predictor of selective laser trabeculoplasty efficacy. Acta Ophthalmol. 2016;94(7):692-6. 13. Anderson DR; Normal Tension Glaucoma Study. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003;14(2):86-90. 14. Schultz NM, Wong WB, Coleman AL, Malone DC. Predictors, resource utilization, and short-term costs of laser trabeculoplasty versus medication management in open-angle glaucoma. Am J Ophthalmol. 2016;168:78-85. 15. Harasymowycz H, Papamatheakis DG, Latina M, et al. Selective laser trabeculoplasty complicated by intraocular pressure elevation in eyes with heavily pigmented trabecular meshworks. Am J Ophthalmol. 2005;139(6):1110-3. 16. Alm, A. Latanoprost in the treatment of glaucoma. Clin Ophthalmol. 2014;8:1967-85. 17. Prum BE, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern guidelines. Ophthalmology. 2016;123(1):41-111. 18. Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189-206. 19. Saheba H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol. 2012;23(2):96-104. 20. Cohen A, Wong SH, Patel S, Tsai JC. Endoscopic cyclophotocoagulation for the treatment of glaucoma. Surv Ophthalmol. 2017;62(3):357-65. |