Retinoscopy began to be used on a regular basis approximately 100 years ago.1 In the past decade or so, for many practitioners an autorefractor has replaced the use of the retinoscope to objectively determine a patient’s refractive error. However, there are still a variety of patients who are better served by an evaluation using something other than an autorefractor or a phoropter.
Patients who cannot or should not be refracted using an autoreractor or phoropter include those who are physically unable to sit up to an autorefractor or sit behind a phoropter.
Additionally, some individuals have pathology that prevents them from getting an accurate autorefraction or prevents an accurate refraction with a phoropter. Examples of individuals who are better served by retinoscopy, a trial frame refraction or both include those who are physically incapable of raising their head due to spinal degeneration, patients with nystagmus or reduced central vision who need to use eccentric fixation to achieve their best acuity, babies, some patients with special needs as well as patients in wheelchairs and some individuals who have to use sign language or a communication device.
Practitioners will be at a loss on how to accurately determine whether these individuals can benefit from a spectacle correction or a spectacle correction change—unless they are savvy with retinoscopy and trial frame refraction.
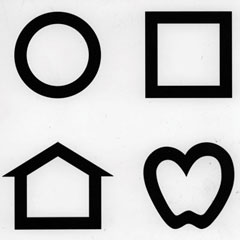 |
| Lea symbols are a must for patients unable to read a traditional visual acuity chart. |
Where to Begin
As practitioners decide between using a phoropter vs. a trial frame, they should consider some of the disadvantages of a phoropter:
- The light reflex for retinoscopy is poorer in a phoropter compared with loose lenses.
- Using multiple lenses in the phoropter decreases light transmission.
- Eccentric viewing by the patient is difficult to impossible with a phoropter.
- Patient with nystagmus struggle to use their null point when looking through a phoropter.
- It is difficult to use just noticeable difference (JND) refraction techniques with a phoropter.
For patients with ocular diseases or physical limitations, using a trial frame will make subjective refractions not only possible, but accurate. The primary advantage of trial frames is that refractions are easier and more natural than with a phoropter for patients who are difficult to refract or those who are visually impaired.
Just Noticeable Difference Refraction Techniques
Clinicians must remember that standard refraction techniques are employed when performing a trial frame refraction on an individual with normal sight. JND refraction techniques are used for those who are visually impaired.2 When in doubt, use the trial frame.
Just noticeable difference is the amount of lens power needed to elicit an appreciable change in clarity or blur. The poorer the visual acuity, the larger the JND.
JND power is determined by taking the denominator of the 20ft Snellen acuity and dividing it by 100. For example, for a patient with 20/200 acuity: 200/100 = 2.00D. Therefore, you would start your subjective refraction with +/-1.00D.
When practitioners use JND refraction techniques, they are better able to do an accurate refraction at any acuity level. JND refracting techniques apply to both sphere and cylinder corrections. Most importantly, JND elicits reliable answers from the patient.
JND Spherical Power Refraction Determination
A patient presents with uncorrected VA of 20/400. There are no old spectacles and the practitioner is unable to do retinoscopy due to significant band keratopathy. To determine JND sphere power, divide 400/100 = 4.00D, and start with +/-2.00D.
If the patient states +2.00D is clearer, put +4.00D in the trial frame. With +4.00D in the trial frame, again asked the patient to compare +2.00D/-2.00D. If the patient still prefers +2.00D, replace the +4.00D lens in the trial frame with a +8.00D lens. With +8.00D in the trial frame, again asked the patient to compare +2.00D/-2.00D. If the patient now prefers -2.00D, replace the +8.00D lens in the trial frame with a +6.00D lens. Check VA, which is now 20/200, making the JND lens +/-1.00. Now the practitioner can refine with +1.00D/-1.00D bracketing lenses. If the patient’s acuity continues to improve, you will eventually fine tune with +0.50D/-0.50D.
 |
| For patients who cannot read, Lea paddles can help clinicians test visual acuity. |
JND Cylinder Power and Axis
Finding the best cylinder axis and power requires the same JND technique described above, now using a Jackson cross cylinder (JCC).
- For 20/50 or better vision, use a +/-0.25D JCC
- For 20/63 to 20/100, use a +/-0.50D JCC
- For 20/125 to 20/160, use a +/-0.75D JCC
- For 20/200 or less, use a +/-1.00D JCC
- JND Cylinder Power and Axis
After establishing the patient’s spherical power as describe above, VA is 20/200; 200/100 = 2.00D, so start with a +/-1.00 JCC.
With the JCC oriented for power at 90/180 degrees, ask the patient which is clearer. If the patient states that -1.00D axis 180 is clearer, put a -2.00D axis 180-cylinder lens in the trial frame. Remember to adjust the sphere power by adding +1.00D to maintain the circle of least confusion on the retina. Now, with a -2.00D axis 180-cylinder lens in the trial frame, again ask the patient to compare +1.00D to -1.00D axis 180. If the patient still prefers -1.00D axis 180, replace the -2.00D axis 180-cylinder lens in the trial frame with a -4.00D axis 180-cylinder lens.
With the -4.00D axis 180-cylinder lens in the trial frame, add an additional +1.00D sphere in the trial frame. Again ask the patient to compare +1.00D to -1.00D axis 180. If the patient now prefers +1.00D axis 180, replace the -4.00D axis 180-cylinder lens in the trial frame with a -3.00D axis 180-cylinder lens and adjust the sphere power by adding -0.50D sphere power. If the patient’s VA has improved to 20/100, refine with a +0.50D/-0.50D JCC. Once the cylinder power is determined, repeat the process to determine the cylinder axis using the standard technique of 15 degree interval change in axis until reversal, then five to seven degree interval change in axis if the patient’s VA allows for this discrimination.3 Remember to recheck the cylinder power with any significant change in the cylinder axis.
A closer look at several cases will help illustrate the benefits of refracting with a retinoscope and trial frame. These cases will also highlight nonconventional VA testing techniques used to aid the subjective refraction.
Case 1: Significant Pathology
An 81-year-old Ethiopian who is unable to read and does not speak English presents with a history of reduced vision. Through an interpreter, we learn that he has not been able to see for any near vision tasks for nine to 10 years. He states he was told he had macular degeneration and cataracts and was informed to not consider cataract surgery due to his macular degeneration because it would limit his potential for improved vision.
 |
| When matching visual acuity is not possible to quantify entrance visual acuity, clinicians can use Teller cards. |
Because this patient is unable to read, VA testing was accomplished via matching with Lea symbols. Uncorrected acuity measured 1/100 (20/2000) OD, 0.5/1000 (20/4000) OS and 1/100 (20/2000) OU. Near acuity measured 30M @ 1’ (1M is equivalent in size to newsprint).
The patient states he would like better walking around vision, which could not be enhanced with a spectacle correction. Additionally, there was no improvement possible with his near vision using optical or electronic magnification devices. In addition to the cataracts and macular degeneration, the clinical examination revealed climatic droplet keratopathy and pseudoexfoliation with normal pressures.
In an effort to enhance this patient’s overall visual functioning and quality of life, cataract surgery was performed with the placement of an IOL followed by penetrating keratoplasty to address the climatic droplet keratopathy for the right eye. Uncorrected VA improved in the right eye following these procedures to 1/80 (20/1600).
The next step was to refract the patient for best-corrected vision. Because the patient could not fixate for an accurate autorefraction assessment, we used retinoscopy to find he was plano -5.00 x 083 with resultant acuity of 2/63 (20/630) and 6.4M @ 6”. This was a 3x improvement in distance vision compared with his pre-cataract surgery vision. No improvement was found in near vision with any powered reading correction. However, the patient was very happy with the improved walking around vision he had when he received his single vision spectacles, which was his primary goal.
Case 2: Low Vision
A 48-year-old non-verbal male presented for evaluation with a history of autism and high myopia, as well as a retinal detachment in his right eye 20 years ago. He had cataract surgery with an IOL 15 years ago, which dislocated 10 years later, leaving him functionally aphakic in his right eye. The left eye is anophthalmic and the patient has no spectacles. He was referred for an evaluation by his care facility to determine current level of visual abilities for programming and appropriate expectations.
 |
| A trial frame set is a must when working with patients who don’t do well with a phoropter. |
Because matching VA was not possible to quantify entrance visual acuity, we used Teller preferential viewing acuity. Teller acuity found the patient able to preferentially view down to the 0.86 cycles per centimeter card at a 50cm working distance. This is equivalent to a Snellen acuity of 20/800. With this level of acuity, he was not able to view the fixation target in the autorefractor. Retinoscopy found +6.75D sphere. This improved acuity by Teller to 1.6 cycles per centimeter (~20/400). The patient was prescribed a single vision Rx to see if it would help him with his activities of daily living, including his ability to see sign language and with social interactions. A month after the patient recieved his new glasses, his care facility reported that he was wear them daily. They noted he was more visually aware of his environment and now no longer needed to use hand-over-hand sign language.
Case 3: Post-surgery
A 58-year-old male was referred by his cornea specialist for a refraction following a penetrating corneal transplant. Severely irregular astigmatism was noted by keratometry and autorefraction results were inconsistent. Uncorrected acuity was 10/160-2. Retinoscopy found +3.50 -9.75 x 162. Manifest refraction found +3.75 - 10.25 x 161, which improved distance vision to 20/50-2+1. Based on his excellent visual potential with high cylinder, his remaining interrupted sutures were removed. Follow-up refractive assessment one month later found +0.50D sphere with resultant acuity of 20/40-2+2.
Case 4: Physical Limitations
A 67-year-old female with severe scoliosis presents with a chief complaint of an inability to read with her recently prescribed bifocals. She is wheelchair bound and cannot raise her head without assistance due to her spinal curve. Corrected visual acuity was 20/25 OD and 20/20 OS. Near acuity through her current spectacle correction was 2.5M continuous text print at 18”. Because this patient could only look down, it was clear she was not able to use the bifocal portion of her spectacles. With her reading correction in a trial frame, she was able to easily read 0.5M continuous text print and the newspaper. Given her physical limitations, she was switched from a bifocal to two single vision Rxs, one for distance and one for near. With a single vision reading Rx, she was able to resume reading with ease.
 |
| Clinicians should use standard refraction techniques when performing a trial frame refraction on an patient with normal sight. |
Case 5: Mental Limitations
A 76-year-old female with Alzheimer’s disease presented for evaluation following the loss of her spectacles at her care facility. Initially, it was felt that because of her lower functioning secondary to the Alzheimer’s disease, she didn’t need spectacles. However, it was noted that, functionally, she was doing much worse with ambulation since losing her spectacles. Additionally, she was having more difficulty seeing to eat.
These observations prompted her referral for an evaluation to see if there was a beneficial prescription. Throughout her visit, she was inattentive, singing children’s songs and did not respond to any form of subjective or objective visual acuity testing. Autorefraction was not possible because the patient would not hold steady for Rx determination. With this in mind, retinoscopy was performed to determine a tentative Rx. Retinoscopy was done a second time over this Rx in a trial frame to refine sphere, cylinder and axis. The final prescribed Rx was for significant hyperopia and astigmatism and, of course, presbyopia. Follow up found her back to baseline for ambulation and the ability to see when eating with the new spectacles.
Case 6: Congenital Complications
A 22-year-old male with a history of reduced vision secondary to oculocutaneous albinism type II with resultant nystagmus presented with a complaint of distance and near vision blur. He had refused spectacles in the past, but recently noted poor vision with work-related tasks. For example, he complained of difficulty identifying distant objects, and he stated he was unable to read for more than a few minutes without significant asthenopia. Uncorrected distance VAs were 20/250 OD, 20/125-2 OS and 20/125-1 OU. At near, he was able to read 0.63M continuous text print at 4”. This patient had a significant anomalous head position with left head tilt and chin-down position. Both the phoropter and autorefraction were challenging secondary to increased nystagmus when he had to position his head in primary position. Trial frame refraction found +1.00 -2.50 x 035 OD with vision improved to 20/125 and +1.00 -2.00 x105 with vision improved to 20/100 OS. Using a 4.00D add, the patient could read 0.50M continuous text for extended periods of time, comfortably and efficiently.
For individuals with nystagmus, remember to fog the non-tested eye by adding plus to decrease vision by two to three lines in that eye. This is a better alternative to occlusion because covering one eye can significantly increase nystagmus amplitude, frequency or both. Additionally, research shows correcting refractive error, especially when significant, can be one of the most effective treatments for vision impairment secondary to nystagmus.4 A careful and accurate refraction has the potential to improve the visual function of patients with both acquired and congenital nystagmus more than other therapeutic interventions, including surgery, medication and prism.4
 |
| Using a Jackson cross cylinder, clinicians can find a patient’s best cylinder axis and power during trial framing. |
For patients who present with conditions that make determining refractive error by autorefraction or with a phoropter challenging, clinicians can turn to tried-and-true retinoscopy. Thus, maintaining your retinoscopy and trial frame refraction skills can make the difference in your ability to help all of your patients see their best.
Use trial frame refraction when visual acuity is 20/50 or worse, or if regular refraction techniques are not successful. For many patients with vision problems, the only intervention needed to enhance their visual functioning is the prescription of appropriate spectacles.
Dr. Shahid is a clinical assistant professor in the department of Ophthalmology and Visual Sciences at the University of Iowa’s Carver College of Medicine, where she provides vision rehabilitation and primary eye care.
Dr. Wilkinson is a clinical professor in the department of Ophthalmology and Visual Sciences at the University of Iowa’s Carver College of Medicine. He is also director of the institution’s Vision Rehabilitation Service.
|
1. Riviello M. When the retinoscope ruled. Rev Optom. 2016;153(7):26-31. 2. Appel SD, Brilliant RL. The low vision examination. In: Essentials of Low Vision Practice. Brilliant RL, ed. Woburn, MA: Butterworth-Heinemann; 1999:19-46. 3. Wilkinson ME. Sharpen your subjective refraction technique. Rev Optom. 2016;153(1):58-65. 4. Hertle R. Examination and refractive management of patients with nystagmus. Surv Ophthalmol. 2000;45(3):215-22. |

