 |
Q:
I have a patient with severe limbal stem cell deficiency (LSCD) who was told by her cornea specialist that she is at high risk for corneal transplantation and her only option is a keratoprosthesis. What is her prognosis with this procedure? What are the risks associated with it? Does she have any other options?
A:
“There are multiple facets to this question,” according to Scott G. Hauswirth, OD, who practices and teaches in Colorado, “but it is important to understand the basic premise behind this dilemma.” He says a high-risk transplant is usually defined as one that has a vascularized cornea or a history of multiple grafts. Immunologic rejection can occur in up to 70% of these grafts, even with aggressive, local immunosuppressive therapy.1
Limbal Territory
The limbus is the border between the cornea and the sclera and is typically 1mm to 2mm wide. It contains a variety of cells with various functions, including the limbal stem cell niche, which is home to the progenitor cells that eventually differentiate and migrate across the cornea to form the layers of the corneal epithelium. Dr. Hauswirth notes that insult to this area decreases its ability to regenerate a healthy corneal epithelium and disrupts the barrier function of the limbus, without which the cornea would become repopulated by conjunctiva, leading to stromal haze, vascularization, opacification and scarring.
Unfortunately, corneal transplantation in patients with LSCD is often destined to fail and represents a significant challenge to practitioners and surgeons.2-4
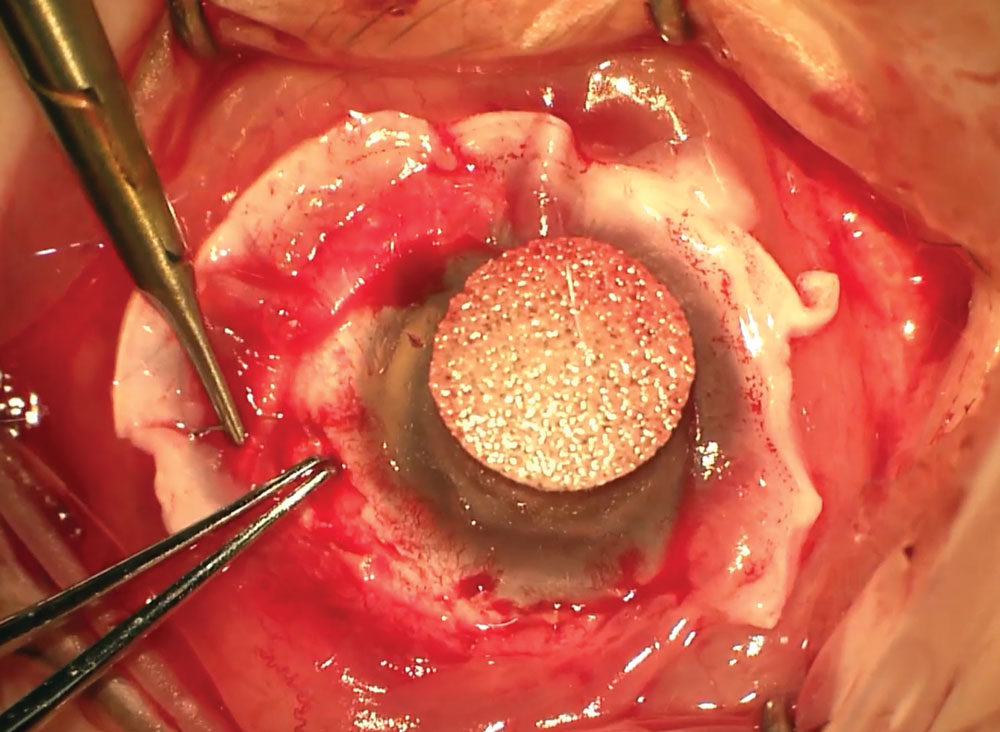 |
This patient is undergoing limbal stem cell transplantation. |
Choose Wisely
According to Dr. Hauswirth, there are two methods to address this challenging case—keratoprosthesis or limbal stem cell transplantation, followed by penetrating keratoplasty. While both are viable choices, he says the decision to choose one over the other depends on the number of previous corneal transplantation attempts and the degree of scarring and loss of viable limbus. Surgeon comfort and experience also play roles, he adds. It’s worth noting that the limbal stem cell transplantation option typically only works well if the patient does not have severe dry eye.3
Multiple studies show good results for keratoprosthesis in eyes with LSCD.5-7 One large literature review noted that 64.1% of eyes reached visual acuities better than 20/200 with a device retention rate of 88.9% over an average follow-up period of 25 months.8 Similarly, a study comparing the results of Boston Type I keratoprosthesis implantation in patients with or without LSCD revealed a device retention rate of 75% and visual acuities of at least 20/200 in 77% of patients in the LSCD cohort.5 Outcomes of patients who underwent limbal stem cell transplantation and penetrating keratoplasty were demonstrated in a study of 48 eyes with LSCD—90% of which were considered high-risk—that achieved a three-year graft survival rate of 62.5%.4
Dr. Hauswirth says keratoprosthesis has evolved to solve the problems presented by multiple graft failures, high-risk grafts and LSCD. He notes, however, that creating a device with biologically inert material that can be incorporated into the ocular tissues to replace the cornea is a novel approach that has taken many different revisions to even come close to perfecting. Dr. Hauswirth adds that while Boston type I keratoprosthesis is the most common in the United States, other methods, such as tibial bone keratoprosthesis and osteo-odonto-keratoprosthesis, have performed well in clinical trials, and some, including AlphaCor keratoprosthesis, are available but have had a less robust uptake in the United States.9
According to Dr. Hauswirth, limbal stem cell transplantation techniques differ based on the origin of the transplanted tissues and their placement location on the eye but usually involve the use of systemic immunomodulatory medications that all have their own set of risks.10 He notes that these stronger local and systemic immunomodulators keep the host immune system from attacking the new limbal cells, ensuring corneal graft survival.
Dr. Hauswirth says the limbal cells can be harvested through autologous cultivation (from a presumably healthy second eye), living donor cultivation or allogenic donor cultivation. In the case of allogenic harvesting, ABO/HLA tissue matching is preferred.11 He notes that while the cells can also be harvested from a cadaver with or without ABO/HLA matching, these patients would likely then be on long-term immunosuppression. After harvesting a 1mm to 2mm section of the limbus, Dr. Hauswirth says the cells are then cultivated on organic media until they reach a size where they can be directly transplanted to the host.
On the horizon are methods that involve transplanting stem cells derived from other areas, such as the oral mucosa, and ex vivo methods of stem cell cultivation, including mesenchymal stem cell harvesting.12,13 However, these methods are rather new and do not have a long track record of success like the two discussed earlier, both of which are worth looking into.
| 1. Jabbehdari S, Baradaran Rafii A, Yazdanpanah G, et al. Update on the management of high-risk penetrating keratoplasty. Curr Ophthalmol Rep. 2017;5(1):38-48. 2. Dua HS, Saini JS, Azuara-Blanco A, et al. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48(2):83-92. 3. Sacchetti M, Rama P, Bruscolini A, et al. Limbal stem cell transplantation: clinical results, limits and perspectives. Stem Cells Int. 2018. 4. Borderie VM, Levy O, Georgeon C, et al. Simultaneous penetrating keratoplasty and amniotic membrane transplantation in eyes with a history of limbal stem cell deficiency. J Fr Ophthalmol. 2018;41(7):583-91. 5. Aravena C, Bozkurt TK, Yu F, et al. Long-term outcomes of the Boston type I keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2016;35(9):1156-64. 6. Basu S, Taneja M, Narayanan R, et al. Short-term outcome of Boston type 1 keratoprosthesis for bilateral limbal stem cell deficiency. Indian J Ophthalmol. 2012;60(2):151-3. 7. Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2011;30(11):1187-94. 8. Shanbhag SS, Saeed HN, Paschalis EI, et al. Boston keratoprosthesis type 1 for limbal stem cell deficiency after severe chemical injury: a systematic review. Ocul Surf. 2018;16(3):272-81. 9. De la Paz MF, Salvador-Culla B, Charoenrook V, et al. Osteo-odonto-, Tibial bone and Boston keratoprosthesis in clinically comparable causes of chemical injury and autoimmune disease. Ocul Surf. April 12, 2019. (Epub ahead of print). 10. Ballios BG, Weisbrod M, Chan CC, et al. Systemic immunosuppression in limbal stem cell transplantation: best practices and future challenges. Can J Ophthalmol. 2018;53(4):314-23. 11. Cheung AY, Sarnicola E, Kurji KH, et al. Cincinnati protocol for preoperative screening and donor selection for ocular surface stem cell transplantation. Cornea. 2018;37(9):1192-7. 12. Choe HR, Yoon CH, Kim MK. Ocular surface reconstruction using circumferentially-trephined autologous oral mucosal graft transplantation in limbal stem cell deficiency. Korean J Ophthalmol. 2019;33(1):16-25. 13. Yazdanpanah G, Jabbehdari S, Djalilian AR. Emerging approaches for ocular surface regeneration. Curr Ophthalmol Rep. 2019;7(1):1-10. |

