40th Annual Technology ReportFollow the links below to read the other articles from our 40th annual Technology Report: Downsize Your Technology to Enhance Your Practice Modernize Your Exam of Glaucoma Patients and Suspects Understanding Today's OCT Technology—And Anticipating Tomorrow's |
Managing age-related macular degeneration (AMD) is a significant focus in primary care optometry, and no responsibility of ours is more urgent than identification. Though we may comanage active disease with the retina specialists who administer anti-VEGF injections, our most vital activity is to maintain vigilance for AMD in vulnerable patients. However, many cases go overlooked due to the absence of structural findings. In fact, a recent study reveals that both optometrists and ophthalmologists miss AMD approximately 25% of the time.1 In addition, 30% of the undiagnosed eyes in that study had large drusen, a known risk factor for wet AMD.1
There’s plenty that optometrists can do to help patients with early-stage disease. Clinically, many have undetected functional deterioration that could yield to diagnostic testing. With an early diagnosis, ODs can take potentially life-altering steps long before patients hit the intermediate stage and are forced to struggle with vision loss. The longer clinicians can keep these patients from advancing to wet AMD and needing injections, the better off they will be.
This article describes the value of diagnosing AMD in its earliest stages using functional tests and how you can bring these methods into your clinic today.
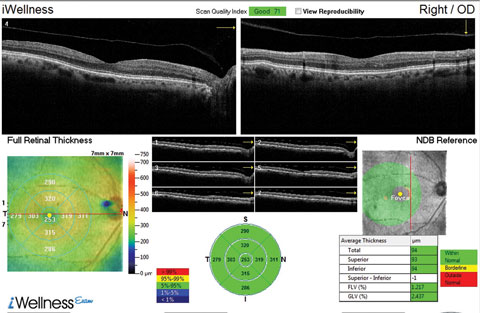 |
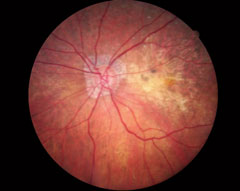
| This OCT screening shows a patient who displayed no macular pathology but for a detached vitreous face. The macular exam showed no visible drusen. However, this patient did have early functional signs of AMD when we tested dark adaptation. Click image to enlarge. |
Disease Before Drusen
Optometrists have a wealth of outstanding technologies available, such as optical coherence tomography (OCT), which is tremendously helpful in imaging an array of retinal diseases, including AMD. However, OCT looks only at structure. In fact, OCT’s great precision in visualizing structural change has likely tipped the priorities of eye care providers more toward this vein of diagnostic data at the expense of monitoring the patient’s functional visual status. Depending on the interpretation, an OCT image of a patient with a few small drusen may be misleading and can potentially underestimate the extent of disease. By the time photoreceptor ellipsoid layer thinning is visible on OCT, macular function is likely greatly impaired.2-4
Research Shows AMD is Going UnnoticedA recent study reveals how frequently dilated eye exams miss incidents of AMD.1 The cross-sectional study, which included 1,288 eyes in 644 adult patients who were enrolled in the Alabama Study on Early Age-Related Macular Degeneration (ALSTAR), revealed that doctors miss AMD about 25% of the time.1 The authors set out to determine the extent to which AMD is underdiagnosed by ODs when the disease is actually present. In the study, they reviewed the medical records of adults 60 years or older.1 To be eligible, the patient’s medical record from the most recent comprehensive dilated examination could not indicate a diagnosis of AMD in either eye, nor could the medical record notes contain terms that signified the signs of AMD. Each patient in the ALSTAR study had digital color fundus photos taken, which were reviewed by masked, trained graders who determined the presence or absence of AMD findings according to the Clinical Age-Related Maculopathy Staging system.2 The types of AMD-associated lesions also were noted. The results revealed that one in four eyes studied was not diagnosed with AMD during the dilated fundus examination, despite having macular characteristics indicative of AMD in the fundus photos. Approximately three quarters of the 320 undiagnosed eyes had 10 or more small drusen (77.8%) or intermediate drusen (78.1%), or both, and 96 (30.0%) of the undiagnosed eyes had large drusen. As this study reveals, even the most well-trained primary eye care doctors can easily miss AMD. Missing this diagnosis can have serious consequences for patients and result in severe vision loss. Also, the prevalence of undiagnosed AMD in the study was not any different between ophthalmologists and optometrists. Although the investigators of this study admit the reasons for the missed diagnoses remain unclear, they point out that improved AMD detection strategies may be necessary in primary eye care offices, since many of these patients would have been candidates for therapeutic intervention. In short, current technology may lead to a sense of complacency and does not always enable us to detect AMD early enough. This is potentially overcome when dark adaptation testing is available.
|
But some technologies are showing promise for more advanced testing. For example, functional testing can detect dark adaptation impairment before AMD is clinically evident, which is helpful considering the disease appears to manifest through impaired dark adaptation before drusen are visible.7,8 In a recent study, subjects with impaired dark adaptation were twice as likely to develop clinically evident AMD and eight times as likely to advance beyond the earliest stage of AMD.7 Some clinicians find reduced contrast sensitivity to be another early signal of incipient disease.
Usually expressed as “night vision difficulties,” impaired dark adaptation is often among the first detectable consequences of AMD and a method of identifying patients with potential subclinical disease.8 Dark adaptation can be used to evaluate whether small drusen are focal deposits or the visible tips of the lesions caused by AMD.8
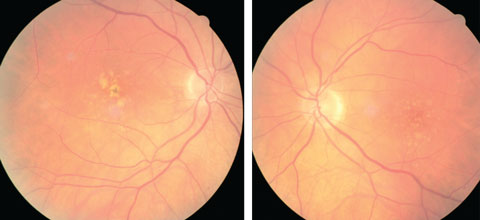 |
| At left, this eye qualifies as intermediate AMD per the AREDS classification system. At right, the patient qualifies as intermediate AMD and is progressing to the advanced stage due to the pigment clumping centrally. Click image to enlarge. Photo: Julie Poteet, OD |
A nuanced understanding of the histopathology of this vision-depleating disease, combined with a keen knowledge of its functional consequences, will provide optometrists the clinical inght necessary to fight AMD earlier than ever before.
Histopathology
Research shows that retinal pigment epithelium (RPE) cells deposit locally generated cholesterol beneath the RPE cell layer and in Bruch’s membrane before drusen are formed.2 With disease progression, this cholesterol continues to accumulate, resulting in focal areas that are sufficiently thickened enough to be identified as drusen.2-4 Essentially, drusen caused by AMD are merely the “tip of the iceberg” of the earliest lesions caused by AMD.2-4 And more dysfunction is present than is apparent based on the clinical or even OCT appearance of drusen.2-4
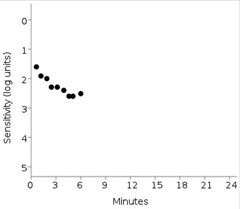 |
| This print out shows the failed dark adaptation screening test of the same patient imaged using OCT above. Anything greater than 6.5 minutes is indicative of impaired dark adaptation time. Also, the patient’s fixation rate was tracked at 0% in this patient. Click image to enlarge. |
This cholesterol accumulation causes three primary insults to the retina: (1) inflammation, (2) oxidative stress and (3) disruption of oxygen and nutrition supplied to the outer retina.3 For instance, transport of vitamin A—critical for rod-mediated dark adaptation—is a functional role disrupted by AMD.3 Research shows that this vitamin A availability disruption dramatically slows dark adaptation.4
Additional research shows dark adaptation function is impaired from the earliest stages of AMD, retinitis pigmentosa and other retinal degenerations/dystrophies, with increasing impairment as the diseases progress.1,4,5 By prevalence, the most common disease which causes dark adaptation impairment is AMD and second is retinitis pigmentosa, which is typically diagnosed before the age of 50.1,4,5
How Adaptometry Works
Dark adaptation testing may allow clinicians to detect subclinical AMD at least three years earlier than it is clinically evident, according to investigators.4,5,9 Unlike macular pigment optical density (MPOD) or genetic testing, dark adaptometry does not measure the risk of AMD; it is diagnostic of AMD, meaning that the disease is already present.
Dark adaptation is a rod-mediated measure, using the rod intercept (RI), and is not dependent on cones. RI is the number of minutes it takes for the eye to adapt from bright light to darkness and can be measured with the commercially available AdaptDx dark adaptometer (Maculogix). Obtaining a patient’s RI is easy both from a technical perspective and for the patient. A bleaching flash is used and then responses to stimuli are recorded until the RI is achieved. Like an automated visual field, all the technician needs to do is encourage fixation and attention. The patient’s experience is similar to a threshold visual field and may take anywhere from a few moments to approximately 20 minutes.
Opinion: Get Off the “Stage”If we could easily identify patients and improve outcomes, why isn’t every doctor doing it? AMD labels and definitions are partially responsible for the current atmosphere of confusion and have prevented us from looking critically at ineffective practice protocols. For example, consider the limited value of the clinical staging of AMD in optometric practice. If, like me, you were taught that “Stage 1 AMD” is “No AMD,” be aware that this is dangerously misleading. Stage 1 AMD is, in fact, AMD. Even though noticeable central vision loss has not yet occurred at this early stage, it one day may, and by the time that day comes heroic measures will be necessary. The clinical staging of AMD is misinterpreted and has led to a defeatist view of what we can achieve in terms of more effective management of dry AMD. By the time we can visualize change, damage has already been done. |
The RI number provides a clear and objective measurement of retinal function with 90% sensitivity and specificity.10 An RI of less than 6.5 minutes indicates normal dark adaptation consistent with healthy photoreceptor function.10 An RI higher than 6.5 minutes indicates impaired dark adaptation, most often due to AMD, unless there is evident retinal degeneration or a systemic vitamin A deficiency.10
Helping Early AMD Patients
Many changes to an AMD patient’s lifestyle can help avoid further central vision loss and retinal damage. Once diagnosed with early AMD, optometrists can encourage patients to take the following steps:
More frequent examinations. Assuming that nothing can be done to effectively intervene at the earliest of stages of AMD ignores the value of informed follow-up. Although more research is needed to most effectively address subclinical AMD, patients with early stage disease should still be monitored more closely because the transition from dry to wet AMD can happen rapidly and, if left untreated, can lead to vision loss fairly quickly.
 |
 |
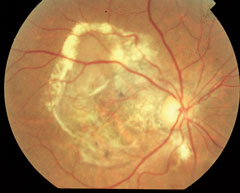 |
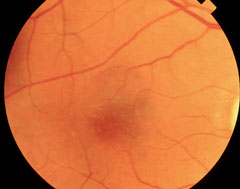
| At top, this patient shows signs of early AMD associated with drusen and areas of RPE disruption. This patient is already at risk for vision loss. With dark adaptation, ODs may be able to begin management of AMD before this clinically evident stage is even reached, in the hopes of preventing progression to the advanced stages of AMD, as seen in the lower two images. Click image to enlarge. Photos: Paul Karpecki, OD, and Diana Shechtman, OD |
Moving from a 12- to a six-month follow-up interval is useful for monitoring disease progression. More frequent visits provide increased opportunity to detect choroidal neovascularization (CNV) before visual acuity loss. Often, home monitoring by Amsler grid is ineffective, or the patient defers reporting symptoms between office visits. ODs can also consider shortening the follow-up visit interval to every three or four months for patients who are fast progressors or are at high risk of CNV. As a result, AMD patients are likely to benefit from a much better outcome than they would have without being closely monitored.
Stay healthy. Following a healthy diet, exercising regularly and maintaining overall health are sound goals for all patients.11 Counseling patients on lifestyle changes is easy and impactful. For example, smokers must be informed of the dramatic escalation of consequences tied to their habit. Much like someone who’s been diagnosed with prediabetes might choose to make dietary changes, most patients diagnosed with AMD will want to take action. With some guidance, clinicians can empower patients to take meaningful steps in the right direction.
One study found that women who followed a healthy diet, engaged in physical exercise and avoided smoking had substantially lower risk of early AMD compared with women who did not follow these healthy lifestyles.12Epidemiological studies have found substantial benefit from higher dietary intake of foods rich in omega-3s, especially DHA.13 A Mediterranean diet is another consideration, as studies suggest those who regularly consume a Mediterranean-style diet carry an overall lower risk of developing advanced AMD compared with those who regularly consume a traditionally Western diet.14
Advocate for an active lifestyle. Research shows this can reduce the risk of progression to CNV.15-17 One study found those who participated in cardiovascular exercise of any intensity three or more times per week had one third the incidence of CNV compared with those who exercised less than three times per week.17 For those who walked one or more blocks per day, the incidence of CNV was half compared with those who walked less than one block a day.17
Recommend supplements and blue light-blocking lenses. Although AREDS showed no benefit to the use of AREDS supplements for patients who have less than intermediate AMD, further research on this specific population is needed.20-22 Some doctors may also recommend carotenoids, omega-3 and blue light-blocking lenses.20-22 These recommendations may not only prove beneficial in slowing disease, they may also improve visual function.5,7 More importantly, an early diagnosis of AMD provides our patients the freedom to choose whether they want to invest in supplements. Evidence strongly suggests that patients should be prescribed nutritional supplements because, on average, treated patients have better outcomes than untreated patients.9,16,17
Opinion: Use New Techniques, but Keep the OldBy Jay M. Haynie, OD Dark adaptation does allow optometrists to measure the functional component of macular disease, including AMD; however, in my opinion, a patient who performs poorly on this test should not necessarily be offered AREDS supplements. Doctors can use dark adaptation testing as a factor in determining risk and to educate patients about AMD monitoring and prevention. Nutritional supplements have been a part of eye care for decades now and we have some great scientific data on the type of patient that may benefit from supplements, such as AREDS, and which patients showed little to no benefit in the larger studies—embracing new testing techniques doesn’t change that. Evidence shows AREDS supplements have been beneficial for patients with phenotypic findings, such as medium-to-large drusen, although more research is needed on whether they can truly prevent the onset of clinical AMD. In addition, investigations raise controversy regarding the use of high levels of zinc contained in supplements. Furthermore, if a patient has a genotype which includes two complement factor H alleles and no ARMS2 allele, zinc may actually contribute to the progression of the disease process.1 This prompts the question as to whether nutritional supplements which contain zinc should be advised for patients who have a low dark adaptation performance. There has been enough controversy regarding the antioxidant zinc that prior to putting a patient on a supplement for what may be decades we must think about potentially doing harm to those individuals. Dark adaptation does have a role to play in optometry and it could be used as a screening tool for patients who have a strong family history of AMD, patients who have subjective symptoms specific to night vision loss, or patients with phenotypic changes suggestive of early AMD (small or intermediate drusen). Upon conclusion of the test, if the patient is found to have poor dark adaptation, my clinical theory and thoughts in private practice would be to begin counseling them about lifestyle changes. These discussions must include the modifiable risk factors of AMD which include good daily nutrition, use of ultraviolet protection, cholesterol reduction, blood pressure monitoring and tobacco cessation. If either the doctor or the patient is considering a supplement, I recommending starting with a carotenoid.
|
Three primary options for the selection of an appropriate nutritional supplement exist. The first is to prescribe a macular pigment supplement (i.e., the xanthophylls: lutein, zeaxanthin, meso-zeaxanthin). The second is to prescribe a supplement containing both xanthophylls and antioxidants, including zinc and vitamins E and C (e.g., an AREDS2 supplement).
The third option is to prescribe a xanthophyll supplement to patients with subclinical and early AMD and a xanthophyll-antioxidant combination supplement to patients who have or progress to intermediate AMD.
The relative merits of each option are debatable, and knowledge continues to expand about the many factors that contribute to AMD progression.
Timely referral to a retina specialist. Early knowledge that AMD is present can trigger a change in management. For example, follow-up visits are likely to include careful funduscopic examination, fundus photography and OCT scans, as necessary, and repeat dark adaptation testing to gauge for progression.23
This careful approach helps ensure patients are referred to a retinal specialist for more aggressive treatment, such as injections, as soon as it becomes necessary.
With an earlier diagnosis, optometrists can do more than let AMD run its course and eventually rob patients of their sight. Though no one diagnostic tool is sufficient on its own, given the complexities of AMD’s pathophysiology and the wide variety of presentations possible in the preclinical phase of development, adding these tools and techniques to our practice allows ODs to keep a closer eye on progression and respond promptly to changes. Improved outcomes begin in the optometrist’s chair—it is well within optometry’s scope of practice to preserve AMD patients’ vision and, ultimately, improve outcomes.
Dr. Gerson practices at Grin Eyecare in Olathe, Kan.
|
1. Neely D, Bray K, Huisingh C, et al. Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 2017;135(6):570-75. |

