Ocular Surface HealthCheck out the other feature articles in this month's issue:- Lid Wiper Epitheliopathy: What the OD needs to know - Red Eye Remedies: New and Tried-and-True - Artificial Tears: What Matters and Why - A Modern Approach to Meibomian Gland Dysfunction (earn 2 CE credits) |
Treating dry eye disease (DED) often raises more questions than it answers, for both doctors and patients. After all, the disease is difficult to understand and even harder to explain. The Tear Film and Ocular Surface Society’s (TFOS) 2017 Dry Eye Workshop II (DEWS II) report defines dry eye as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.” That definition, while thorough and elegant, leaves a lot for us to unpack with our patients.1
The complex, multifactorial nature of the disease makes treating it feel like pulling on the thread of a sweater—the many facets of the disease are woven together, and uncovering one starts to unravel the whole thing. The cascade of “whys” can be a challenge for optometrists to explain and difficult for patients to accept. But an educated patient is a motivated patient, and success often hinges on compliance. Here, we discuss the dry eye questions patients often have and how clinicians can keep patients focused on what’s important: improving their ocular health.
Editor’s note: This article was planned and written while Dr. Hauser was a private practitioner. By the time of publication, Dr. Hauser had become an employee of Novartis Pharmaceuticals Corporation; however, the company did not review or influence the content and no products are discussed.
 |
|
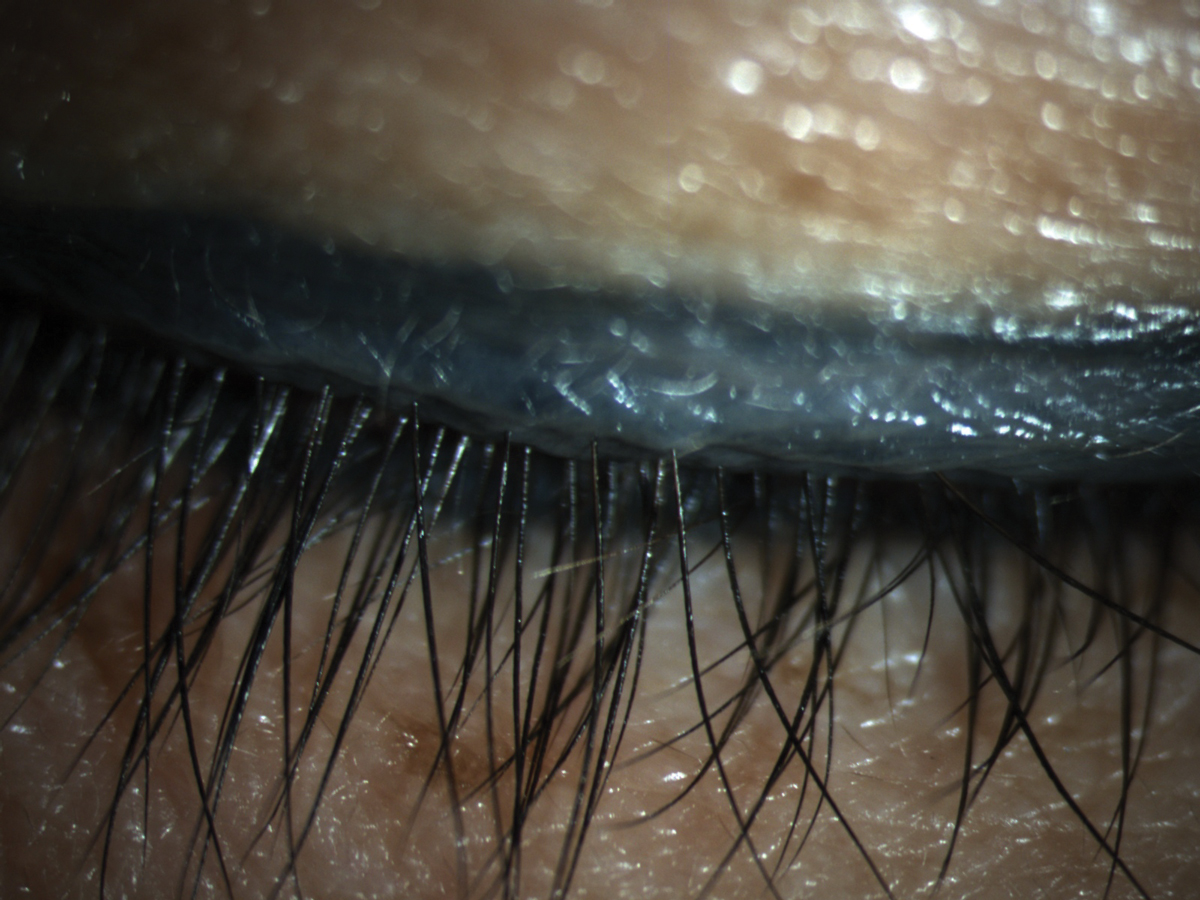
Patients require extensive education on the importance of removing makeup properly to help reduce dry eye symptoms related to MGD. Click image to enlarge. |
Why Me?
This used to have a straightforward answer: because you are a woman over the age of 50 or you have an autoimmune or other systemic disease.2 However, the DED patient profile is evolving. While older women continue to dominate the dry eye demographic (with a prevalence of 17.9% vs. 10.5% in men), clinicians are reporting larger numbers of men and younger patients.3 These emerging demographic trends may cause practitioners to reassess how they answer the question, “Why me?”
The complexity of dry eye makes it difficult to explain to patients why they have the condition. Risk factors are not only variable in their total number but also in their frequency, as some lifestyle and environmental risk factors can wax and wane with seasons or the patient’s visual, occupational and lifestyle demands. Demographics (i.e., age, sex, race), meibomian gland dysfunction (MGD), connective tissue disease, Sjögren’s syndrome, androgen deficiency, hematopoietic stem cell transplantation, environmental conditions and medication use are all consistent risk factors (Table 1).3-6
Another commonly associated risk factor is dermatologic rosacea, which can manifest with ocular complications—including dry eye—in up to 72% of patients.7 Menopause and fluctuating hormone levels remain controversial risk factors, although recent research shows low androgen levels are associated with increased DED, and hormone therapy in postmenopausal women can increase the risk of dry eye by as much as 70% for estrogen users and 30% for those taking estrogen+progesterone/progestin.2
The “why me” is difficult to answer for patients because many have multiple risk factors for DED. As doctors begin to explain the patient’s specific risks, these tend to accumulate into a compendium, and “why me” transitions to “of course you” as the physician works their way through the list and inevitably hits a few. Whenever possible, try to prioritize just a few key risk factors in your discussion to help the patient retain as much as possible.
Table 1. DED Risk Factors3,5,6 | |
| Risk Factor | Odds Ratio |
| Age (18 to 34 as reference) | |
| 35 to 44 | 1.29 |
| 45 to 54 | 1.95 |
| 55 to 64 | 3.34 |
| 65 to 74 | 3.74 |
| Older than 75 | 4.95 |
| Race (white as reference) | |
| Asian | 1.08 |
| Hispanic | 1.34 |
| African American | 0.96 |
| Other | 1.44 |
| Chronic conditions | |
| Arthritis | 1.59 |
| Osteoporosis | 1.70 |
| Allergies | 1.81 |
| Thyroid disease | 1.62 |
| Migraine headache | 1.69 |
| Medication use | |
| Antihistamine | 1.65 |
| Steroids | 1.84 |
| Antidepressants | 1.44 |
| Hormones (women) | 1.54 |
| Contact lens wear | 2.14 |
| Female sex (male as reference) | 1.88 |
Why Do I Need to Change My Habits?
Once patients understand the underlying causes of DED, a good question to pose back to them is, “What can you do?” Although many demographic features and systemic associations are unavoidable, the patient’s hygiene habits, environment and medication regimen all provide an opportunity for adjustment. While making a change to a single variable in the equation may appear inconsequential, it could make an immeasurable improvement in the patient’s quality of life.
Environment. A crucial—and often modifiable—environmental factor today is increased digital device use. Many patients reluctantly acknowledge their screen time is higher than ever before. Even if they are unable or unwilling to decrease the time spent on digital devices, clinicians can help patients incorporate healthy screen time habits to reduce the risk of dry eye. In addition to modern influences such as digital device use, airflow and low humidity levels are also environmental triggers that can cause an increase in symptoms.
Blinking is key to maintaining the integrity of the ocular surface, and several studies have identified a reduction in blink rate with computer use.8 One team found a mean rate of 18.4 blinks/minute that decreased to 3.6 blinks/minute with computer use. With an approximate 80% decrease in blink frequency, it’s not surprising that patients will have accompanying dry eye symptoms such as irritation, burning and intermittent blur.8 Most doctors now recommend the 20-20-20 rule for screen use: every 20 minutes, look at something 20 feet away for 20 seconds.
Hygiene. Patients also need to learn how to better care for their eyelids. Researchers note that as many as 86% of patients with dry eye also have some form of MGD.9 The condition is characterized by duct obstruction, abnormal (or even non-existent) gland secretion and gland atrophy.10 The symptoms of this dysfunction are the hallmarks of dry eye: eye fatigue or pain, dryness, gritty sensation, itching, redness and blurred vision, to name a few.10
To avoid this complication, patients should be caring for their eyelids the same way they care for their teeth. Optometrists can use the dental model as an analogy to help patients understand the importance of lid hygiene. Lid wipes and warm compresses can help keep the eyelids clean and healthy, just as brushing keeps teeth clean and disease-free. Deeper in-office dental cleanings are likened to in-office thermal treatments for the eyelids.
 |
|
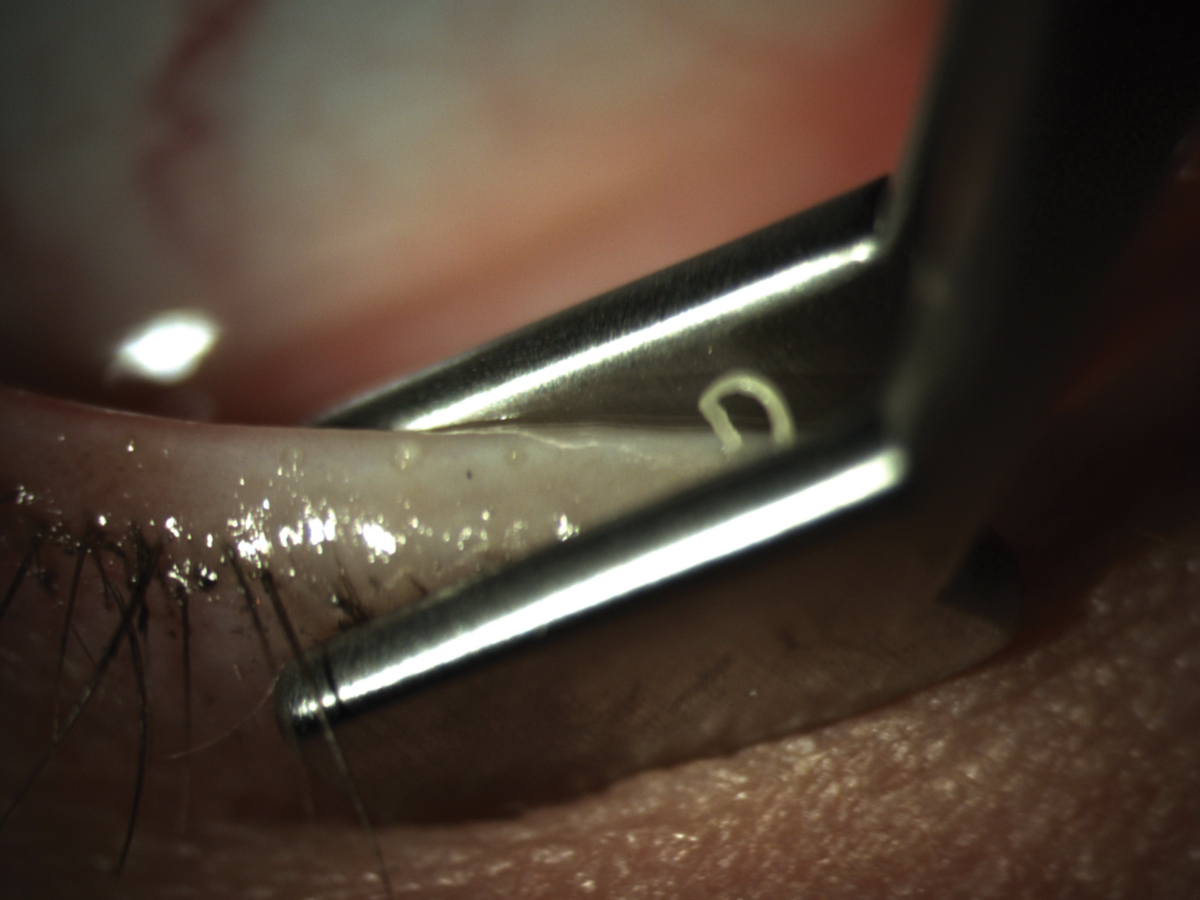
MGD patients will have abnormal, if any, gland secretion upon expression. Click image to enlarge. |
Unfortunately, patients often understand what doctors are saying but don’t necessarily embrace it. Most adults have been exposed to the necessity of good oral hygiene since they were children. Optometrists, however, are often beginning the lid hygiene conversation at the patient’s mid-life when it is arguably the most difficult to change behavior patterns. Even once clinicians provide a reasonable rationale for ocular hygiene, they must remember that mid-life behavior changes are not easily executed no matter how cogent the argument. Patience is required with multiple educational touch points.
The drivers behind non-compliance are not black and white and are unique to each patient. Like DED itself, non-compliance tends to be multifactorial. Physicians can be guilty of over-simplifying why patients fail to adhere to recommendations rather than trying to better understand why they don’t. Lack of adequate patient education could be chief among the reasons, but compliance issues can also be a result of a poor physician-patient relationship, limited patient involvement in decision-making, physical barriers and others.11
Therapies. The well-known association between systemic medications and ocular surface disease needs to be investigated with each patient and, if need be, their primary care provider or other prescribing physician. If a likely culprit is found but there’s no practical way to replace it with an ocular surface-sparing alternative, explain to the patient the potential need for palliative artificial tear use or other mitigating strategies. If prescription dry eye therapy is warranted, explain that the goal is to interrupt the vicious cycle of inflammation so that other mitigation efforts can have time to take hold.
Blinking and ThinkingWhile several studies have found decreased blink frequency with digital device use, clinicians must keep in mind that increased cognitive demand may also play an important role in the altered blink frequency. Higher cognitive load (such as more challenging reading material) can worsen the impact of existing stressors, such as low contrast text and uncorrected refractive error. One team of researchers presented 16 teenagers with text written on a modern tablet computer and a hard copy printed version. The study found the mean blink rates for the low demand task were 8.34 and 9.06 blinks/minute for the computer tablet and the paper versions, respectively. When the subjects were presented with material requiring increase cognitive demand, the mean blink rates decreased significantly to 7.43 and 6.67 blinks/minute. The study suggests that technological advances in digital displays may more closely replicate printed materials.1
|
Why Won’t it Go Away?
That’s a valid question—with a complicated answer. Notably, the DEWS II definition includes no mention of the chronicity of the disease. When the TFOS members were updating the definition of DED, they elected to retain some of the original language of the foundational definition, such as the loss of homeostasis and significant etiological roles of inflammation and hyperosmolarity. The consensus also broadened the definition to include neurosensory abnormalities as one of the additional etiologies. While we don’t fully understand the mechanisms behind these abnormalities, their role in DED is undeniable, and their inclusion in the updated definition was important for differentiating DED from other ocular surface diseases.1
Interestingly, the term chronic is mentioned only once in the Definition and Classification section of TFOS DEWS II.1 The report recognizes that the chronic and progressive nature of DED is often described clinically, but the committee considered the current evidence insufficient to include chronicity in the definition.1 The report does, however, emphasize the vicious and cyclic nature of DED, with the many drivers of ocular surface inflammation feeding into one another.12
Other sources support the foundational DEWS II definition, but further categorize DED into chronic and episodic. Episodic dry eye tends to be triggered by environmental or visual tasks that result in a reduction in blink rate and tear film stability, causing dry eye symptoms. Although chronic dry eye can be exacerbated by the same environmental or ergonomic provocations, it persists with symptoms and possible damage to the ocular surface.13
The chronic nature of dry eye tends to have parallels with other chronic conditions, such as the inflammatory forms of arthritis (non-inflammatory variants also exist). Chronic dry eye is also an inflammatory condition with symptomatic exacerbations precipitated by activity. It can impede the patient’s quality of life and cause varying degrees of pain. Both diseases have effective treatments, but no cure.
The prevalence of arthritis between 2013 and 2015 was approximately 54.4 million in the United States, a number projected to rise to 78 million by 2040 (26% of the US population).14 Similarly, approximately 34 million Americans are symptomatic for dry eye, and the TFOS DEWS II report notes that the global prevalence can range anywhere from 5% to 50%—and as much as 75% of patients over the age of 40.3,15,16
Because public awareness of arthritis may be more widely accepted than DED, clinicians can use the analogy as an educational tool when discussing the “why” of the chronic nature of dry eye. Just like inflammatory joint pain, DED may require daily treatment or medication to quell the symptoms. In spite of daily mitigation, exacerbations may occur and require additional short-term management.
 |
|
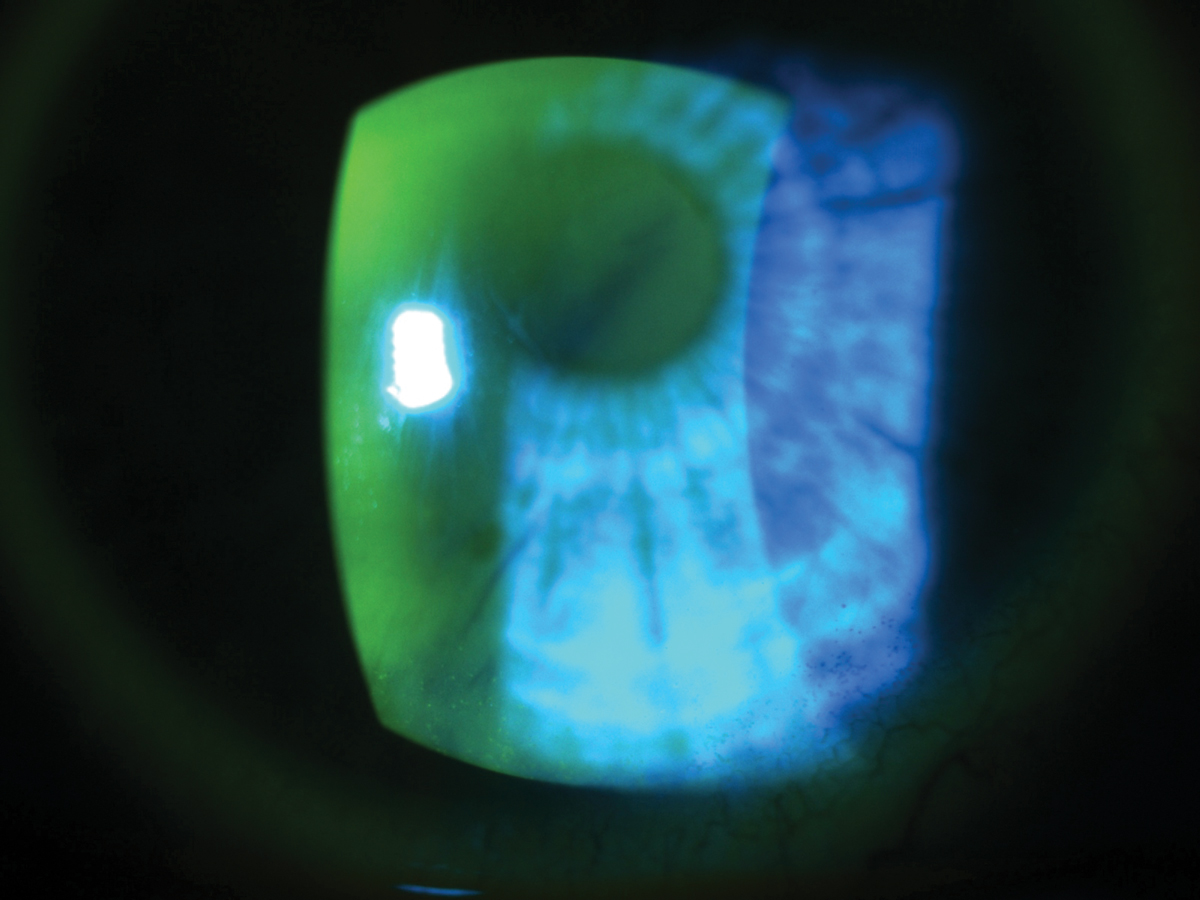
Dry eye patients need ongoing patient education and encouragement to stay the course of treatment and self-care. Click image to enlarge. |
Why Are You Asking About My Feelings?
When managing DED, practitioners are often desensitized to the impact the diagnosis and the subsequent management has on the patient; however, it is life-altering.
Eye care providers may consider DED a less significant diagnosis than other chronic, progressive diseases such age-related macular degeneration or glaucoma, because it rarely poses a threat to vision. However, patients who hear that their eye irritation and vision fluctuations can’t be solved with an updated prescription will be understandably upset. They have a chronic condition without a cure that, if severe enough, requires attention and behavior change for the foreseeable future.
Research shows DED comes with a significant burden, both physically and financially. It can impair physical and mental functioning and, in moderate-to-severe disease, can have symptoms equivalent to severe migraine.17 A recent study found that for each 10-unit difference in Ocular Surface Disease Index scores, participants’ work and activity were impaired by 4.3% and 4.8%, respectively.18
A 2011 study found dry eye patients in the United States spend approximately $11,302 treating their disease.19
Depression and anxiety are widely accepted comorbidities of DED, and the diagnoses are often intertwined. The diagnosis of chronic illness and the management of chronic pain can induce a “stress state” that can aggravate both the disease and a patient’s depressive tendency. Similarly, patients on depression medications, such as selective serotonin reuptake inhibitors, for reasons unrelated to eye health can experience dry eye side effects. In recent years, several studies have established overlap between pain- and depression-induced neuroplasticity changes, as well as neurobiological changes.20
Researchers note that patients with DED are also more likely to be diagnosed with dementia, bipolar disorder, depression and neurotic disorders.21 They further speculate that DED and depression may have similar inflammatory etiologies, as DED patients have increased production of inflammatory cytokines in the tears and conjunctiva, while those with depression have high levels of the same inflammatory cytokines and neuropeptides in the blood.21
In addition, the medical management of depression can have sicca effects, especially in at-risk patients. Research shows medications with anticholinergic effects, such as tricyclic antidepressants, can cause decreased lacrimation.22,23
When patients ask the many whys of dry eye, be prepared with answers as unique as the people asking them. Canned responses don’t inspire trust, compliance or loyalty to the practice—an individualized approach that takes into consideration each patient’s risk factors, hygiene habits and medical history will.
Dr. Hauser is director of peer education for ophthalmics at Novartis. She continues to practice clinical care in limited capacity at The Eye Specialty Group in Memphis, Tenn., with a focus on ocular surface disease and surgical management.
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-83. 2. Matossian C, McDonald M, Donaldson KE, et al. Dry eye disease: Consideration for women’s health. J Womens Health (Larchmt). 2019;28(4):502-14. 3. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the Beaver Dam Offspring Study: Prevalence, risk factors, and health-related quality of life. Am J Ophthalmol 2014;157:799-806. 4. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802-12. 5. Ward MF, Le P, Donaldson JC, et al. Racial and ethnic differences in the association between diabetes mellitus and dry eye disease. Ophthalmic Epidemiol. 2019;26(5):295-300. 6. Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. 2017;182:90-98. 7. Oge’ LK, Muncie HL, Phillips-Savoy AR. Rosacea: diagnosis and treatment. Am Fam Physician. 2015;92(3):187-96. 8. Sheppard AL, Wolffsohn JS. Digital eye strain: prevalence, measurement and amelioration. BMJ Ophthalmol. 2018;3:e000146. 9. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-8. 10. Chhadva P, Goldhardt R, Galor A. Meibomian gland disease: the role of gland dysfunction in dry eye disease. Ophthalmology. 2017;124(11S):S20-S26. 11. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1(3):189-99. 12. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. 13. Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405-12. 14. Hootman JM, Helmick CG, Barbour KE, et al. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 2016;68(7):1582-87. 15. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334-65. 16. US Census Bureau. Current Population Survey, Annual Social and Economic Supplement, 2008. Accessed May 1, 2020. 17. Galor A, Levitt RC, Felix ER, et al. Understanding the true burden of dry eye disease. Expert Rev Ophthalmol. 2015;10(5):403-5. 18. Greco G, Pistilli M, Asbell PA, Maguire MG. Association of severity of dry eye disease with work productivity and activity impairment in the Dry Eye Assessment & Management Study. Ophthalmology. October 14, 2020. [Epub ahead of print]. 19. Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30(4):379-87. 20. Sheng J, Liu S, Wang Y, et al. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast. 2017;2017:9724371. 21. Liang C, Cheang W, Wang C, et al. The association of dry eye syndrome and psychiatric disorders: a nationwide population-based cohort study. BMC Ophthalmol. March 20, 2020. [Epub ahead of print]. 22. Malone DA Jr, Camara EG, Krug JH Jr. Ophthalmologic effects of psychotropic medications. Psychosomatics. 1992;33(3):271-7. 23. Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents. CNS Drugs. 2010;24(6):501-26. |

