13th Annual Pharmaceuticals ReportCheck out the other feature articles in this month's issue: Steroid Wars: New Drugs Challenge Old Habits |
For patients with dry eye disease (DED), treatment options were once confined to artificial tears, warm compresses and the occasional off-label steroid. While some of these help soothe symptoms, they do little to truly manage the disease, especially as it worsens. For patients no longer treatable with these at-home remedies, new research shows a cascade of discoveries in both diagnosis and treatment.
Today, optometrists can treat these patients with prescription topical and oral anti-inflammatory medications, point-of-care treatments, heating and expression of glands and even advanced options such as amniotic membranes. While these advances are giving our patients a new lease on ocular comfort, optometrists have much more to navigate. Applying the most appropriate treatment still varies widely within optometric practices.3
While no clear consensus exists on prescribing dry eye drugs, understanding how these medications work can help doctors tailor effective management for each patient.
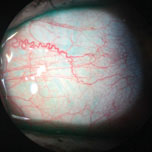 |
| This patient’s inflamed blood vessels can be seen without any vital dyes. Photo: Vin Dang, OD. Click image to enlarge. |
The Loss of Homeostasis
When it comes to treating DED patients, timely and effective options can be complicated by the need to accurately detect and identify underlying etiologies. In 2017, the Tear Film and Ocular Surface Society (TFOS) shed new light on DED with its second Dry Eye Workshop report (DEWS II). That research recasts DED as a multifactorial condition characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms that lead to tear film instability and hyperosmolarity.1 This turned on its head the old paradigm of categorizing DED as either aqueous-deficient dry eye (ADDE) or evaporative dry eye (EDE). With the DEWS II’s conclusions, these are no longer considered completely separate ocular surface conditions, as researchers have found up to 70% of DED patients have mixed etiologies.2 At the forefront of that loss of homeostasis is inflammation.
The TFOS DEWS II report also taught us “in DED, tear hyperosmolarity is considered to set up a cascade of signaling events within surface epithelial cells, that leads to the release of inflammatory mediators and proteases. Such mediators, together with the tear hyperosmolarity itself, are conceived to cause goblet cell and epithelial cell loss and damage to the epithelial glycocalyx. Damage is reinforced by inflammatory mediators from activated T-cells, recruited to the ocular surface. The net result is the characteristic punctate epitheliopathy of DED and a tear film instability which leads at some point to early tear film break-up. This break-up exacerbates and amplifies tear hyperosmolarity and completes the vicious circle of events that lead to ocular surface damage.”1 This vicious inflammatory circle is a common pathway that all forms of DED enter, regardless of etiology.
Inflammation
On the ocular surface, the acute phase of inflammation results in the degranulation of mast cells and the subsequent release of histamine and phospholipids. The inflammatory stimuli also initiates the degradation of the cell membrane via phospholipase A2 and subsequently leads to the formation of arachidonic acid (AA). The latter is metabolized by 5-lipoxygenase (LOX) and cyclooxygenase isoenzymes (COX-1/COX-2), resulting in the recruitment of white blood cells and the formation of prostaglandins and thromboxane A2, respectively.4,5 Prostaglandins play a role in pain response and increase vessel permeability.
One of the many impacts of this cascade is the production and release of inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-a), interleukin-6 (IL-6) and matrix metalloproteinases (MMPs) by the ocular surface epithelial cells.1,5 This results in the activation of antigen-presenting cells and increased expression of adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and selectins by the conjunctival vascular endothelium.5 This facilitates recruitment of additional inflammatory cells to the ocular surface.
Chronic inflammation involves the processing of antigens by ocular antigen-presenting cells and naive T-cells. Primed CD-4 T-cells adhere to activated vascular endothelium and enter the ocular tissue. Cytokines produced by activated T-cells, such as interferon gamma (IFN-Y), amplify the immune response by increasing adhesion molecule expression by ocular blood vessels.5 Arachidonic acid metabolites such as leukotrienes and prostaglandins are actively involved in the development of inflammatory disease.4
Recognizing the significance and complexity of inflammation in DED gives us the opportunity to identify gaps or overlap in treatment, provided we can identify where each prescription fits along the cascade.
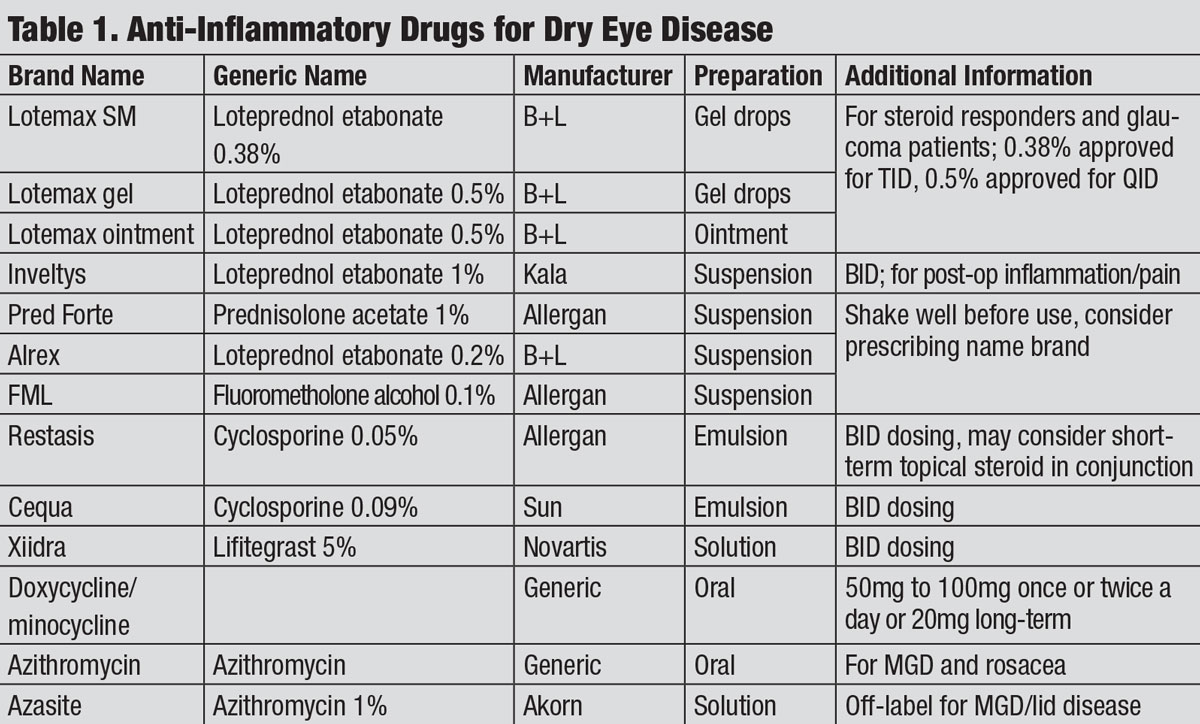 |
| Anti-Inflammatory Drugs for Dry Eye Disease. Click table to enlarge. |
Corticosteroids
These topical meds stimulate the production of a glycoprotein called lipocortin. Formed lipocortin inhibits the activity of phospholipase A2, which releases arachidonic acid and ultimately results in the formation of prostaglandins and thromboxane A2.6 Steroids also inhibit the formation of IL-1, ICAM-1, MMPs and cytokines.6 These actions produce anti-inflammatory and immunosuppressive effects on a localized level as steroids block both the LOX and COX-1/COX-2 pathways of the inflammatory cascade, reducing vasodilation, vascular permeability and stabilizing cellular membranes.7
Clinical trials show significant improvements for patients with moderate to severe ADDE, with improvements in corneal staining and general injection.6 As a result, these medications are often highly effective short-term anti-inflammatory options for patients with ocular surface inflammation.
Choosing the most appropriate corticosteroid for your patient requires knowledge of both the medication’s mechanism of action and your particular patient. From a clinical standpoint, loteprednol has numerous ophthalmic indications and also comes in a wide array of formulations, making it a flexible option for practitioners.8
New versions of topical drug delivery for loteprednol also adds to the appeal. Lotemax SM gel (loteprednol 0.38%, Bausch + Lomb) uses submicron particles to enhance drug dissolution in the tears, which increases the transcorneal penetration compared with Lotemax 0.5% gel drops and enables greater adherence to the ocular surface.9 Furthermore, Lotemax SM is also preserved with a low dose of benzalkonium chloride (BAK) and at TID dosing, reducing the burden on an already compromised ocular surface. For this same reason, in addition to fewer unwanted side effects (elevated IOP and subcapsular cataracts), Lotemax SM is a good short-term anti-inflammatory for patients suffering from concomitant DED and glaucoma.
While not FDA approved for DED management, it has been used as an off-label treatment for many dry eye patients based on the consensus of peer-reviewed professional literature.
Another loteprednol option is Inveltys (loteprednol etabonate suspension 1%, Kala), which attaches loteprednol to a mucus-penetrating nanoparticle to improve penetration and concentration of the drug to ocular tissue.10 Lastly, although not yet FDA approved, is another loteprednol option, KPI-121 0.25% (loteprednol etabonate suspension 0.25%, Kala), that would specifically be approved for signs and symptoms of DED.11
Outside of loteprednol, many alternative options for the treatment of ocular surface inflammation exist, including FML (fluorometholone ophthalmic suspension 0.25%, 0.1%, Allergan) or prednisolone acetate 0.12%. These are available in generic formulations and can be effective in DED management, especially as they cost less than branded counterparts.
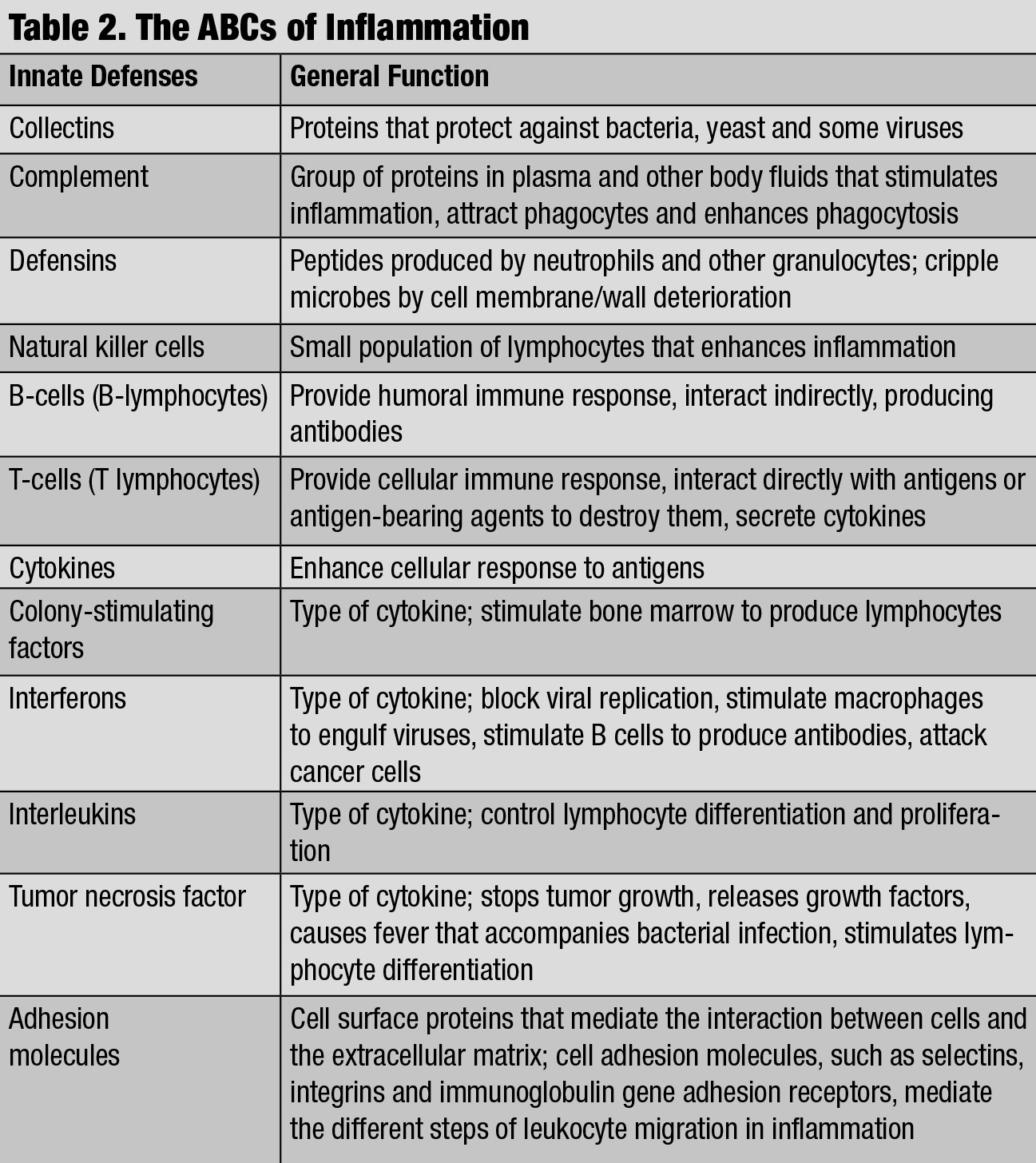 |
| The ABCs of Inflammation. Click table to enlarge. |
Cyclosporine Immunomodulators
Restasis (cyclosporine A 0.05% emulsion, Allergan) is an immunomodulator that acts on T-cells in the tear film, conjunctiva and cornea, and has been available for ophthalmic use since 2003.12 Cyclosporine A (CsA) suppresses inflammation by binding to the protein cyclophilin, which ultimately results in reducing interleukin-2 (IL-2) formulation and suppressing T-cell activation. lL-2 is secreted by T-helper cells and stimulates proliferation of cytotoxic T-cells and additional T-helper cells.12,13
As CsA halts further activation of T-cells, but does not target already active T-cells, there may not be an immediate improvement in DED signs. For this reason, clinicians often prescribe a short dose of corticosteroids upon the initiation of Restasis. An additional source of patient noncompliance arises from the vehicle of drug delivery. CsA 0.05% requires suspension in an emulsifying agent, such as glycerin or castor oil, as it has poor water solubility on its own. This suspension contributes to some of the side effects such as burning stinging and hyperemia.13
Cequa (cyclosporine 0.09% ophthalmic solution, Sun Ophthalmics) was approved by the FDA in 2018 and recently entered the ophthalmic market.14 Dosing with nanomicellar technology (Ncell, Sun Ophthalmics) BID to avoid the solubility issues with CsA 0.05%, Cequa aims to provide the eye with the highest concentration of CsA on the market.14 Nanomicelles possess both hydrophilic and hydrophobic properties, which enhances their ability to effectively penetrate the ocular epithelium with minimal irritation or drug degradation.14 With a similar side effect profile to Restasis, it will have to be seen how patient compliance responds to the higher concentration level of cyclosporine and if concomitant steroid use will be required for effective relief of symptoms.
Other Immunomodulators
Xiidra (lifitegrast ophthalmic solution 5%, Novartis) entered the market in 2016 and is currently the only approved treatment for both signs and symptoms of DED.15 Similar to cyclosporine, Xiidra also inhibits the T-cell mediated inflammatory pathway by preventing recruitment and activation to ocular surface. However, Xiidra works by blocking the interaction between lymphocyte function-associated antigen 1 (LFA-1) and intercellular adhesion molecule 1 (ICAM-1).16
Blocking this interaction of LFA-1on T-cells and ICAM-1 reduces T-cell activation and migration from the blood vessel to the ocular surface, as well as the secretion of multiple pro-inflammatory cytokines (e.g., IL-1, TNF-a, IFN-Y), reducing inflammation.17 Clinical trials of lifitegrast demonstrated statistically significant improvement in signs and symptoms of DED.17 With the preservative-free dosing, the most common side effects are burning, blur upon instillation and dysgeusia. From a pharmacology standpoint, the inflammatory mediators are still present on the ocular surface when Xiidra is initiated, and a steroid could still be employed to enhance patient comfort. That being said, many patients are successfully managed with Xiidra as the first-line or stand-alone treatment.
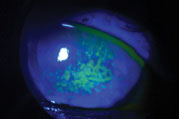 |
| Sodium fluorescein staining shows this patient’s corneal surface is compromised by dry eye disease. Photo: Vin Dang, OD. Click image to enlarge. |
Antibiotics
Oral tetracycline and tetracycline derivatives (e.g., doxycycline, minocycline) can be used to treat DED associated conditions such as rosacea, blepharitis and meibomian gland dysfunction. These broad spectrum antibiotics regulate lipids and inhibit bacterial protein synthesis in addition to having anti-inflammatory properties.2 Research shows they can decrease MMP and phospholipase A2 activity, as well as reduce the production of inflammatory mediators like IL-1 and TNF-a, resulting in reduced irritation and improved tear film stability.2 These features make them an attractive option for short-term management of ocular inflammation or on a long-term basis at a lower dose, with minimal side effects.2
Another antibiotic option for management is oral azithromycin, as the anti-inflammatory properties help control both lid inflammation by inhibiting pro-inflammatory cytokines and bacterial flora.2,18 While no universal agreement on dosing exists, a shorter course of treatment using 250mg to 500mg over five days can be efficacious in rosacea management.18 When it comes to making a prescribing decision for an oral treatment, consider azithromycin as an initial option, unless otherwise contraindicated. A study released in 2019 demonstrated that oral azithromycin efficacy was superior to oral doxycycline for treatment of meibomian gland dysfunction when considering dosing and duration.19
Azasite (azithromycin ophthalmic solution 1%, Akorn) was FDA approved for bacterial conjunctivitis, but has been well-tolerated for off-label treatment of meibomian gland dysfunction and EDE. In addition to the decrease in inflammatory mediators and suppression of proinflammatory mediators, research shows that topical azithromycin can improve lipid behaviors of meibomian gland secretions.20 No research yet shows any benefit to combining systemic and topical antibiotics to improve DED treatment.
As recent research shows, DED is complex and challenging. Before ODs can begin to manage it, they require a clear diagnostic understanding of the individual patient’s ocular surface. This means identifying any concurrent ocular conditions as well as systemic factors that may exacerbate disease.
When determining treatment, clinicians must consider osmolarity, inflammation, treatment history and ocular surface staining. That being said, using these indicators, optometrists should prescribe dry eye therapy that includes specific targeting along the inflammatory cascade, especially early on in disease presentation. In addition, approaching treatment and management with flexibility may serve these patients more in the long run. Although off-label options, consider TID or QID CsA dosing, prescribing concomitant anti-inflammatory treatments such as both Restasis and Xiidra, or a CsA treatment along with oral antibiotics to effectively treat and manage patient signs and symptoms. Using multiple therapies and modalities to address symptoms early on may provide that extra advantage.
Ultimately, educating patients and emphasizing the importance of lifelong management for DED is essential. There will be good days and bad days, as well as better and worse seasons of DED, and management modifications are not always setbacks in the disease course.
Dr. Grant is a clinical instructor at the Southern College of Optometry in Memphis, Tenn.
1. Bron AJ, dePavia CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438-510. 2. Jones L, Downie L, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(4):580-634. 3. Craig J, Nelson J, Azar D, et al. TFOS DEWII report executive summary. Ocul Surf. 2017;15(4):802-12. 4. Patil K, Mahajan U, Unger B, et al. Animal models of inflammation for screening of anti-inflammatory drugs: implications for the discovery and development of phytopharmaceuticals. Int. J. Mol. Sci. 2019;20(18):4367. 5. McDermott A, Perez V, Huang A, et al. Pathways of corneal and ocular surface inflammation: a perspective from the Cullen Symposium. 2005;3(4):S131-S138. 6. Pflugfelder S. Anti-inflammatory therapy for dry eye. Am J Ophthalmol. 2004;137(2):337-42. 7. Sheppard J, Comstock T, Cavet M. Impact of the topical ophthalmic corticosteroid loteprednol etabonate on intraocular pressure. Advances in therapy. 2016;33(4):532-52. 8. Fong R, Cavet M, DeCory H, Vittitow J. Loteprednol etabonate (submicron) ophthalmic gel 0.38% dosed three times daily following cataract surgery: integrated analysis of two Phase III clinical studies. Clin Ophthalmol. 2019;13:1427-38. 9. Fong R, Silverstein B, Pece J, et al. Submicron loteprednol etabonate ophthalmic gel 0.38% for the treatment of inflammation and pain after cataract surgery. J Cataract Refract Surg. 2018;44(10):1220-9. 10. Loteprednol etabonate ophthalmic suspension 1% [Prescribing information]. inveltys.com/pdf/INVELTYS_Package_Insert_Aug_2018.pdf. August 2018. Accessed January 3, 2020. 11. Kunzmann K. Potential dry eye therapy KPI-121 receives FDA CRL. www.mdmag.com/medical-news/dry-eye-therapy-kpi-121-fda-crl. August 15, 2019. Accessed February 14, 2020. 12. Cyclosporine ophthalmic emulsion 0.05% [Prescribing information]. www.accessdata.fda.gov/drugsatfda_docs/label/2017/050790s027lbl.pdf. Accessed Dec 29, 2019. 13. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2):119-25. 14. de Oliveira RC, Wilson SE. Practical guidance for the use of cyclosporine ophthalmic solutions in the management of dry eye disease. Clin Ophthalmol. 2019;13:1115–1122. 15. Lifitegrast ophthalmic solution 5% [Prescribing information]. www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/xiidra.pdf. Accessed Dec 29, 2019. 16. Abidi A, Shukla P, Ahmad A. Lifitegrast: A novel drug for treatment of dry eye disease. J Pharmacol Pharmacother. 2016;7(4):194-8. 17. Sun Y, Zhang R, Gadek T, et al. Corneal inflammation is inhibited by the LFA-1 antagonist, lifitegrast (SAR 1118). J Ocul Pharmacol Ther. 2013;29(4):395-402. 18. Voils S, Evans M, Lane M, et al. Use of macrolides and tetracyclines for chronic inflammatory diseases. Ann Pharmacother. 2005;39(1):86-94. 19. De Benedetti G, Vaiano AS. Oral azithromycin and oral doxycycline for the treatment of Meibomian gland dysfunction: A 9-month comparative case series. Indian J Ophthalmol. 2019;67:464-71 20. Foulks GN, Borchman D, Yappert M, et al. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29(7):781-8. |

