As an eager, young practitioner, I (Dr. Cymbor) can recall seeing a patient for the first time. A man, approximately 70 years old, presented with a previous diagnosis of glaucoma. His visual field showed an inferior arcuate in the right eye and scattered nonspecific defects in the left eye. His intraocular pressures (IOPs) were in the high teens on topical medications. Even though his nerves showed minimal cupping, his diagnosis of glaucoma seemed reasonable, and I continued his topical meds. One year later, his fields and nerves were unchanged.
Upon further scrutinizing his nerves, I noticed his right eye had a small amount of pallor corresponding to the field defect and the other eye had a somewhat crowded disc. Further questioning revealed he had suffered an episode of a sudden change of vision in his right eye 10 years prior but never had it checked. One year after that event, he was diagnosed with glaucoma. Based on this new history and stable fields and nerves, I changed his diagnosis to non-arteritic ischemic optic neuropathy, stopped the drops and he has been stable ever since.
Reflecting on this case, I should have questioned the diagnosis of glaucoma. I might have saved him the burden of a year of unnecessary treatment.
Not every patient with a reduced OCT and visual field actually has glaucoma. Non-glaucomatous disorders may mimic glaucoma. Comorbidities may also influence the management of glaucoma. Patients with glaucoma have a higher risk of systemic and retinal disease. One study found that 50.5% of patients with glaucoma also had hypertension and more than 30% had hyperlipidemia or diabetes.1 A meta-analysis also found that patients with glaucoma had higher mean triglyceride levels.2 Patients with these diseases have a higher risk of non-glaucomatous optic neuropathies. Patients with glaucoma are at a higher risk of retinal disease, such as diabetic retinopathy (DR) and age-related macular degeneration (AMD).3
In other words, patients with glaucoma have a higher risk of non-glaucomatous eye disease and patients with non-glaucomatous eye disease have a higher risk of glaucoma.
 |
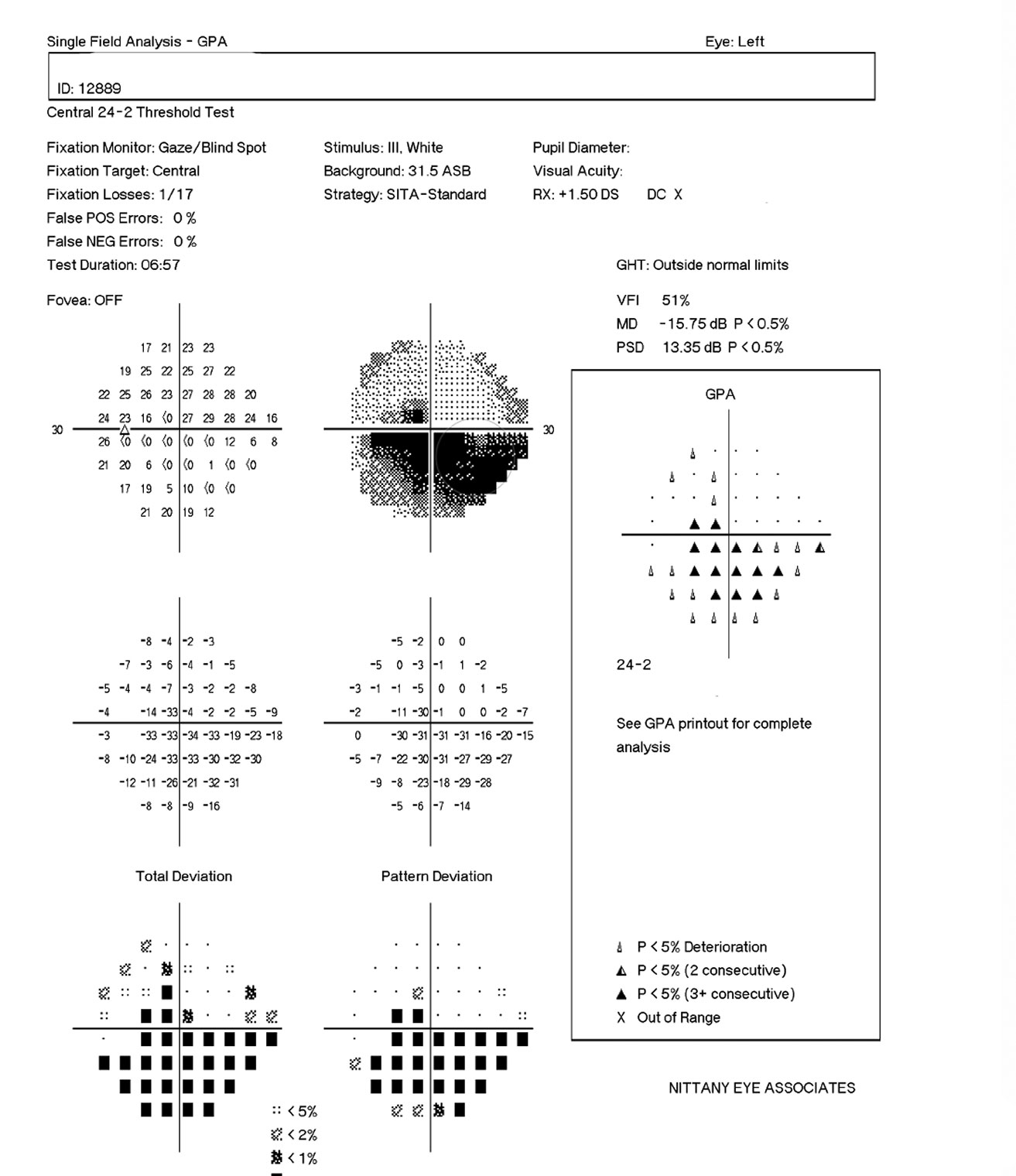 |
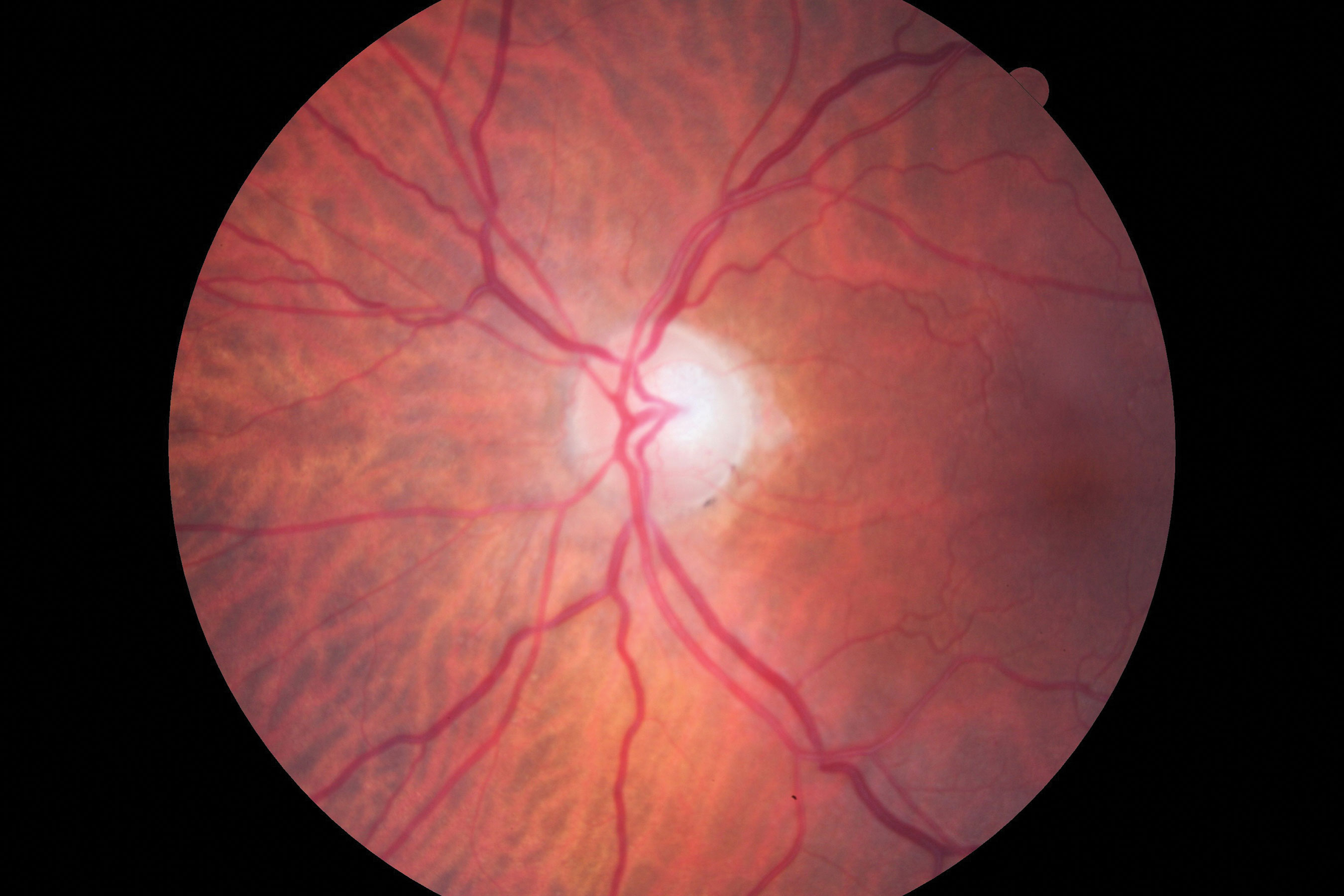
| Figs 1 and 2. Visible in this patient’s optic disc above is a temporal pallor with numerous arterial constrictions and a/v nicking. Below is her visual fields. Click images to enlarge. |
Hypertension and IOP
A relationship exists between systolic and diastolic BP and IOP. A 10mm Hg increase in systolic blood pressure is associated with a 0.26mm Hg increase in IOP and a 5mm Hg increase in diastolic BP is associated with a 0.17mm Hg increase in IOP.5
Patients with hypertension and hypertensive retinopathy have an increased risk of retinal artery occlusion, retinal vein occlusion (RVO), retinal arteriole macroaneurysn, BP, anterior ischemic optic neuropathy (AION), AMD, arteriolar emboli, epiretinal membrane, cystoid macular edema and glaucoma.6 Because of these associations, be careful to distinguish when fundus, field and nerve changes originate from glaucoma from other pathologies.
While RVOs must be monitored for secondary neovascular glaucoma, the later stages of RVOs can also manifest as possible glaucomatous damage to the optic nerve head upon first glance. If a sclerotic or “ghost” vessel is present, ODs can infer ischemic damage if corresponding with a similar area of retina and disc. This would be even more reassuring of a diagnosis in the setting of chronic diseases, such as hypertension, diabetes and hypercholesterolemia. It is when the vessel re-perfuses that confusion can arise.
Optic disc pallor has been found in 20% of those with major branch retinal vein occlusion (BRVO).7 Research shows RNFL thinning of the BRVO-affected area in both ischemic and non-ischemic vein occlusions.8
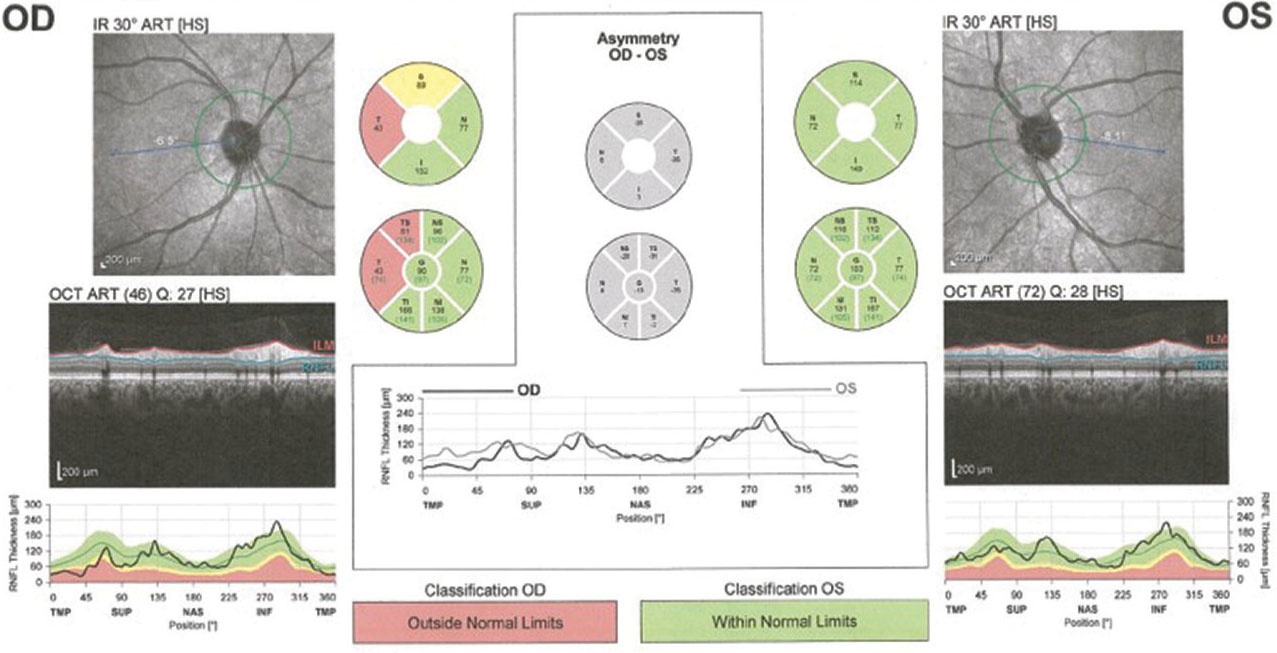
When Comorbidities CollideAn 83-year-old woman came to the office for her yearly evaluation. She had an ocular history of a non-arteritic ischemic optic neuropathy (NAION) OS, which occurred within a few days of cataract surgery six years prior due to a transient IOP spike. She also had a history of dry eye. Her systemic history was significant for longstanding hypercholesterolemia and hypertension. Her best-corrected visual acuity (BCVA) was 20/25 OD and 20/70 OS. IOPs were typically in the mid-teens, but had climbed to 33mm Hg OD and OS at this visit. Central corneal thicknesses (CCT) were 543µm OD and 541µm OS with corneal hysteresis (CH) of 11.7mm Hg and 11.0mm Hg. This lower CH may put the left eye more at risk for glaucoma conversion/progression. Her blood pressure (BP) was 139/85 RAS. The optic disc of the left eye showed temporal pallor with numerous arterial constrictions and a/v nicking (Figure 1). Her visual field showed a dense inferior defect (Figure 2). An OCT image showed a reduced paramacular ganglion cell complex (GCC) with supratemporal atrophy of the retinal nerve fiber layer (RNFL) (Figure 3). This case illustrates the complexity of managing glaucoma with multiple comorbidities. The patient has a previous diagnosis of NAION in the left eye, hypertensive retinopathy in the right and, now, ocular hypertension OU. Although her right eye’s visual field and OCT images were unremarkable, her recently increased IOPs were a major concern, especially in the right eye because of an increased risk of an ischemic event due to the NAION in her left eye. A topical beta-blocker may not be the best option because of her hypercholesterolemia, as beta-blockers may worsen the condition.4 We started her on a preservative-free prostaglandin, which lowered her IOP by approximately 30% to 20mm Hg OS and 21mm HG OD. After several months she reported difficulty with drop insertion, so we performed bilateral selective laser trabeculoplasty (SLT). Her IOPs have since been consistently in the high teens. |
Nonglaucoma Neuropathies
AION is the most common acute optic neuropathy among patients older than 50 and it may be the most easily confused with glaucoma.9 Both produce nerve fiber bundle field defects and nerve fiber layer atrophy. After initial axonal swelling, nerve fiber atrophy occurs and may continue for up to six months before stabilizing.10 OCT images of the GCC show a strong correlation with visual field because GCC thinning from AION occurs prior to nerve fiber layer thinning.11,12
Arteritic anterior ischemic optic neuropathy (AAION) represents inflammation and a vascular occlusion of the posterior ciliary arteries, which originate from the ophthalmic artery, resulting in optic nerve infarction.13 AAION accounts for only 5% to 10% of all AION cases, but half of them develop optic disc cupping.14,15 Patients may experience a rapid onset of unilateral vision loss with a devastating reduction in visual acuity and color vision. Altitudinal visual field defects and relative afferent pupillary defects are common.16 History is key as patients may describe symptoms of jaw claudication, scalp tenderness, fatigue, weight loss and headache. AAION without symptoms—occult AAION—occurs in 20% of cases.17
Nonarteritic anterior ischemic optic neuropathy (NAION) occurs from a small-vessel infarction of the anterior optic nerve in the posterior ciliary arteries. NAION accounts for 90% to 95% of all AION cases and typically affects patients between ages 50 and 70.9,18 Symptoms typically are painless, unilateral and occur immediately or evolve over several hours to days. Visual acuity varies from 20/20 to no light perception but stays better than 20/200 around two-thirds of the time.9 For these patients, color vision is reduced, and a relative afferent pupillary defect will occur in unilateral cases. An altitudinal defect is often seen, but an arcuate defect may also occur, making it more difficult to differentiate from glaucoma. Optic nerve cupping is uncommon in NAION. The non-affected eye often has a crowded disc with the absence of a cup. OCT angiography (OCT-A) may help differentiate between NAION and glaucoma.19
Except for acute angle closure, glaucoma rarely presents with sudden vision loss. AION patients, however, often experience sudden vision loss when waking up. Acute AION patients often have optic nerve edema while nerve edema does not occur with glaucoma. Pallor is another way to differentiate the two pathologies.
In general, glaucomatous eyes have larger and deeper cups, with less rim volume.13 Glaucomatous rim tissue remains pink late into the disease state.
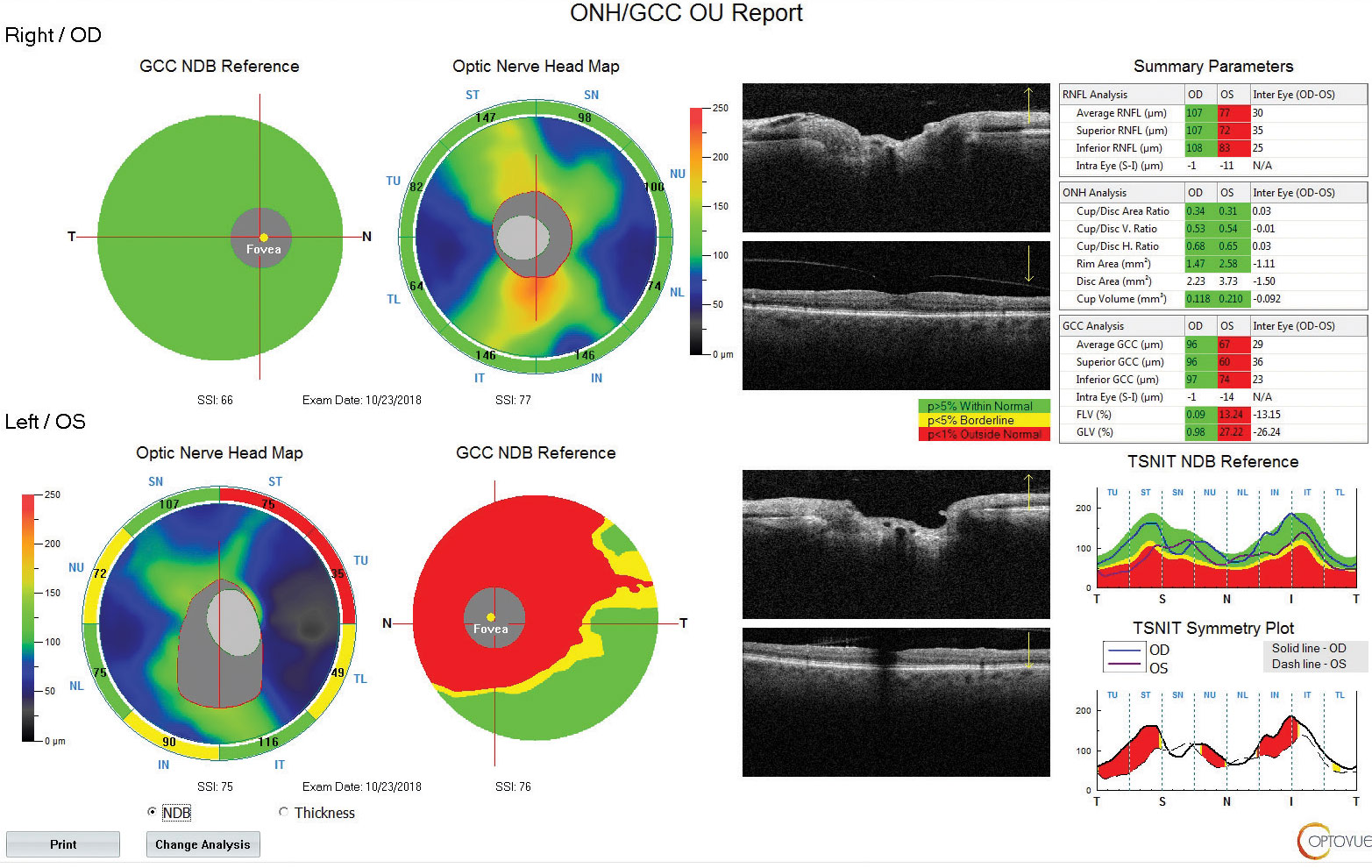 |
| Fig. 3. Using OCT technology, we can see the patient also has a reduced paramacular ganglion cell complex with supratemporal atrophy of the RNFL. Click image to enlarge. |
Neuropathy Etiology
When considering possible causes of optic neuropathy, think about the demyelinating processes that can contribute to these findings. Multiple sclerosis (MS), perhaps the most well-known demyelinating disease, is classically associated with optic neuritis and can occur in up to half of all MS patients.20 This swelling of the optic nerve can result in optic atrophy and pallor with a resultant abnormal OCT six to eight weeks following onset.21
Visual field defects can be unpredictable with this disease. Partial arcuate, paracentral and arcuate nerve fiber bundle defects are the most common defect patterns post inflammation in both the affected and fellow eyes.22 When considering the possible diagnosis of glaucoma in a patient without a known history of MS, carefully assess the history for concurrent neurological symptoms. Peripapillary RNFL thickness is thinner in MS patients.23
Neuro-sarcoidosis and NFL DefectsA 48-year-old black female with a history of sarcoidosis was first diagnosed in 2004. Her most recent angiotensin converting enzyme reading in mid-2018 was normal and her last pulmonary function test around the same time showed mild obstructive lung defects with normal volumes and diffusion capacity. Her first comprehensive eye exam was in 2010 and since that time, her highest measured IOP had been 19mm Hg in both eyes. In 2017, fundus photography showed a superior temporal RNFL defect with corresponding pallor (Figure 4). This NFL damage was further confirmed with OCT (Figure 5). A full glaucoma work-up was completed at that time with normal gonioscopic findings and thin pachymetry (510µm/495µm). RNFL values have remained stable since that time. 24-2 visual field showed central defects in the right eye corresponding on 10-2. This patient is being closely followed for neuro-sarcoidosis due to possible involvement of the optic nerve. Neuro-sarcoidosis affects 5% to 15% of those with sarcoidosis and commonly manifests with Bell’s palsy, auditory impairment, seizures, memory loss, hallucinations and pituitary abnormalities.
|
Tumors
Various other brain malignancies can cause optic neuropathies that mimic that of glaucomatous damage. In these cases, doctors must consider all aspects of the visual pathway.
While vision loss with primary open angle glaucoma is typically gradual and painless, so is that of vision loss associated with optic pathway gliomas. In general, this type of benign tumor typically affects children, but malignant glioblastomas occur in adult males and carry a very poor prognosis.
Approximately 10% of optic pathway tumors occur within the optic nerve and, when labeled as an optic nerve glioma, typically present with unilateral proptosis and afferent pupillary defect.24 Unilateral papillitis will be seen initially, but then leaves the optic nerve atrophic with pallor disproportionate to what would be seen with end-stage glaucomatous cupping.24
When considering the pattern of RNFL loss contributing to increased cupping seen in compressive optic neuropathy, typically more nasal and temporal loss is noted, but any type of cupping is quite rare.23 This is different to what is commonly seen in glaucoma as supra-temporal and infratemporal RNFL loss.
Meningioma is the most common type of tumor of the head and typically affects women as they age.25 This tumor can occur in any area of the brain and accounts for the second most common primary optic nerve tumor. This type of tumor also presents with gradual and painless vision loss, typically unilateral. A pathognomonic feature of this tumor is the formation of optociliary shunt vessels that result from chronic retinal venous outflow resistance.26 While optic disc swelling may also be seen, most often secondary pallor will be noticed.
Blood flow abnormalities must also be considered with optic neuropathies where glaucoma is only one of the differential diagnoses. Along the 50mm course of the optic nerve, direct compression by aneurysm occurs from branches of the internal carotid artery, which maintains the vascular supply to the anterior optic pathway within the circle of Willis. Rupture of the aneurysm can cause ischemia to the tissue it supplies, also leading to atrophy.27 While follow-up radiologic studies assessing blood flow would be warranted, a history of worsening headaches could hint at this lesion.
Several toxins may contribute to optic neuropathy that can resemble glaucomatous RNFL pattern deficits in the setting of a seemingly normal work-up. Typically painless, progressive and bilateral in nature, toxic optic neuropathy can have devastating and irreversible effects. Other common findings include color vision abnormalities, sluggish pupils, temporal optic nerve head pallor and central scotoma. Suspected etiology is that of impaired vascular supply and metabolism due to toxin accumulation within the unique configuration of the blood supply of the optic nerve head.28 Alcohol (methanol), antibiotics, antiarrhythmic, anticancer, heavy metals and carbon monoxide are all possible etiologies of toxic optic neuropathy and lab work (CBC, urinanalysis, metal screening, B12) should be ordered. Neuroimaging is often needed to rule out other causes of optic nerve head pallor. Vitamin B deficiency can amplify the effects of these toxins and contribute to nutritional optic neuropathy, commonly seen in alcoholic patients.29
If presenting at the time of injury, traumatic optic neuropathy could be a straightforward diagnosis. Patients who present years after the event require an extensive history. While, most likely, decreased visual acuity or visual field defects bring these patients into your clinic, objective signs on examination often include optic atrophy due to decreased color vision and afferent pupillary defect testing.
The full glaucoma work-up including gonioscopy is necessary in those patients for whom past ocular trauma is suspected to rule out angle recession glaucoma as a contributor to the impaired optic nerve function. The International Optic Nerve Trauma Study found that 85% of patients with indirect traumatic optic neuropathy were male, with the most common injury etiology being that of motor vehicle accidents. Optic atrophy can take three to six weeks after initial trauma to present, so close follow up is a requirement.30
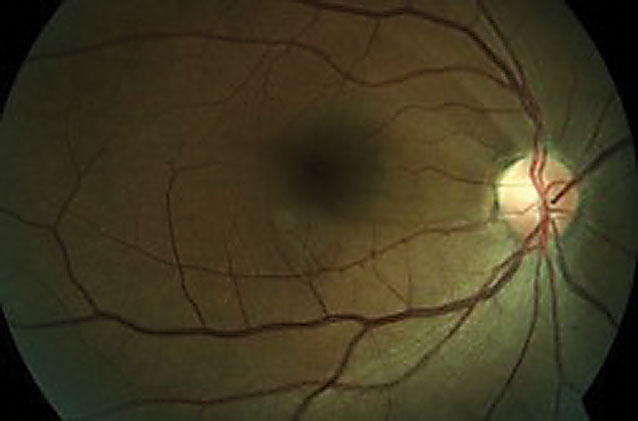 |
| Fig 4. This fundus photo shows a 48-year-old female’s superior temporal RNFL defect with corresponding pallor. |
Infections
Sexually transmitted diseases (STDs) can cause systemic issues within every organ system. Syphilis is known as the “great imitator” as its sequelae can present in any part of the eye and often cause devastating consequences if left untreated. This spirochete infection does not commonly involve the optic nerve (estimated at about 20% of cases), but can be bilateral or unilateral in presentation. Papillitis is commonly noted at initial presentation, which can make the diagnosis difficult if this has cleared and left the nerve atrophic with pallor.31 A history of intravenous drug use, past STDs, or multiple sexual partners can aid in ordering lab work to rule this out.
Rapid plasma reagin typically is used to detect active infection (rule in) while treponemal tests, such as fluorescent treponemal antibody absorption, are ordered to detect if infection has ever occurred, indicating a past exposure.32 Argyll Robertson pupil is a sign of late-stage syphilis where small, bilateral pupils are present that constrict more to a near object than to bright light.31
 |
| Fig. 5. This OCT reading of the same patient from page 58 confirms her RNFL damage. Click image to enlarge. |
Congenital Conditions
Several inherited conditions exist that can result in longstanding or progressive optic atrophy that could leave the clinician with an initial lengthy list of differential diagnoses. Leber’s hereditary optic neuropathy is one of these conditions in which the patient can initially present with a seemingly normal posterior pole. As an example, a 21-year-old Caucasian male presented to the clinic with bilateral blurry vision for the past month. No improvement was shown on refraction with BCVA of 20/100 in each eye. Upon further questioning, the patient stated that his mother and maternal grandfather have never had good vision as well as his male cousin, while his female cousin has been deemed a Leber’s carrier.
Due to the pathology of this optic neuropathy inherited as a mitochondrial disorder, males are disproportionately affected along the maternal lineage. The symmetric and bilateral nature of this patient’s case led to a lack of afferent pupillary defect, but this can occur if asymmetric at presentation. While extensive testing could still be needed to rule out other causes of optic neuropathy and severe vision loss, specifically questioning the ocular history of the patient’s maternal lineage can greatly aid in the diagnosis.
Recent study has focused on the effects of degenerative neurological diseases on the retina, specifically in using OCT to detect early changes indicative of Alzheimer’s disease. Decreased RNFL thickness, increased cupping and vascular tortuosity have all been associated with Alzheimer’s.23 The literature suggests that certain ophthalmologic conditions (AMD, glaucoma and DR) may serve as precursors to Alzheimer’s disease and exist in the same neurodegenerative pathway. One large study found that in those recently diagnosed with glaucoma, a 46% higher risk of Alzheimer disease risk was found.33 This diagnosis should be kept in mind for those showing RNFL changes and possible dementia symptoms.
Mimickers of glaucomatous optic atrophy and RNFL loss can be tricky, especially in the setting of mildly elevated IOP and positive family history of glaucoma. When evaluating a patient where the clinical findings may lead to another diagnosis, one must consider all aspects of past history and current behavior when deciding upon further diagnostic evaluation. While glaucoma could be playing a role in the patient’s optic nerve head appearance and visual field loss, there could be more contributing factors that warrant further treatment for best visual outcome.
Dr. Cymbor is a partner at Nittany Eye Associates in State College, PA. He is also the co-director of the Glaucoma Institute of State College and a member of the Optometric Glaucoma Society.
Dr. Stiles is an Army optometrist practicing at Fort Leonard Wood, Missouri.
|

