Chances are, you’re never going to see a patient with mucormycosis. The rare fungal infection’s incidence is approximately 1.7 cases per million inhabitants per year.1 Still, you can never say never. We saw a case at The Eye Institute at Salus University that nearly evaded us. The propensity of the disease to mimic more common orbital conditions, such as bacterial orbital cellulitis, can result in a delayed diagnosis, which can significantly worsen the outcome. And that worsened outcome can mean some serious consequences for the patient, such as losing vision, the eye itself or even their life.
This article will guide you to a better understanding of this condition and discuss the tools necessary to recognize when an atypical orbital cellulitis includes underlying risk factors of mucormycosis.
Case Report
A 22-year-old female was referred from the emergency department with a chief complaint of vision loss and eyelid swelling of two days’ duration. The patient reported she was also suffering from mental status changes presumed secondary to chronic substance abuse (heroin injection and inhalation). She reported no vision, but pain, in her right eye and a headache. She had no history of previous ocular injuries or trauma but reported having insulin-dependent diabetes mellitus, for which she was properly medicated, with both metformin and insulin, but noncompliant. She denied having allergies of any kind.
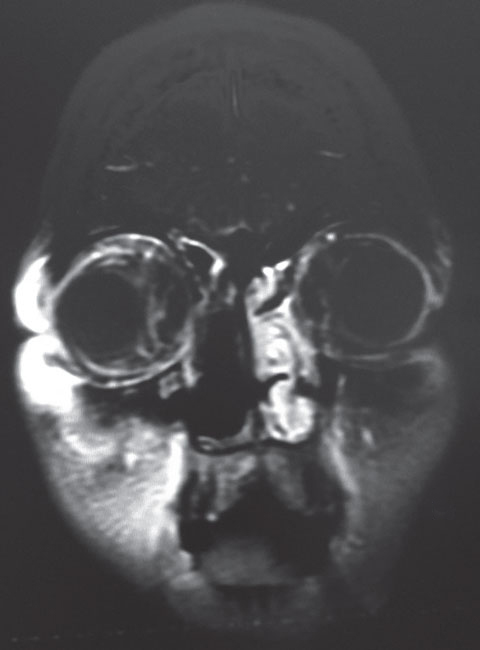 |
| This coronal MRI shows our 22-year-old patient’s orbital inflammation status, post nasal sinus tissue resection. |
Her best-corrected visual acuities were no light perception OD and 20/50 OS. Her left eye exhibited normal motilities while the right eye exhibited complete ophthalmoplegia. She had an obvious right afferent pupil defect. Using biomicroscopy, we found an injected, boggy and edematous conjunctiva in her eye.
Her intraocular pressures (IOPs) were 24mm Hg OD and 13mm Hg OS. The dilated fundus examination demonstrated an ophthalmic artery occlusion with papillitis in her right eye and normal structures with no posterior pathology in the left.
Additional testing at the patient’s bedside included retropulsion of the globe to determine the level of orbital congestion, forced duction testing to establish mechanical resistance to eye movement, Luedde exophthalmometry to record the amount of proptosis and first aid for retinal artery occlusion, which consisted of lowering IOP to increase perfusion along with aggressive ocular pulsed pressure therapy. Additionally, we ordered neuroimaging studies in conjunction with the ear, nose and throat team.
Ultimately, we diagnosed this patient with complete ophthalmoplegia secondary to orbital swelling (orbital cellulitis) induced by mucormycosis infection of the right ethmoid sinus with concurrent ophthalmic artery occlusion.
The Basics
Mucormycosis is a rare, fulminant fungal infection caused by a mold of the order Mucorales.2-5 It is most frequently found as an infection of the nasal and maxillary sinuses that spreads to adjacent structures and causes a condition known as rhino-orbital-cerebral mucormycosis (ROCM).2-4 Though rare, ROCM is the third most common fungal infection behind Candida and Aspergillus, with its incidence continuing to rise most likely due to increased diagnostic accuracy.4
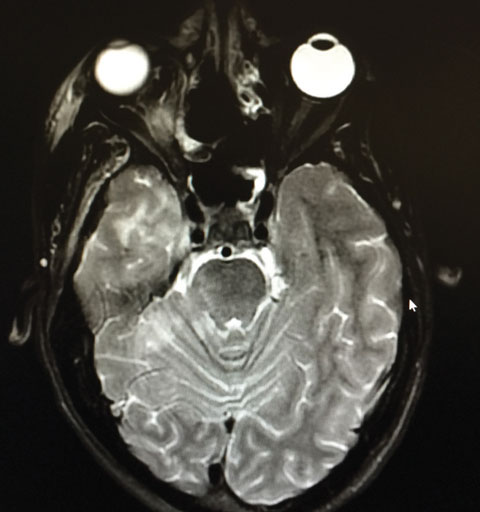 |
| Our patient’s axial MRI shows residual orbital congestion and inflammation post extensive sinus tissue resection. Click image to enlarge. |
Researchers have identified several significant risk factors for onset of ROCM, including: uncontrolled diabetes, immunosuppression, excess serum iron levels and ketoacidosis.2,3,6-9 Often, some form of breakdown of the physical barriers of the immune system are present in mucormycosis patients; for example burns, surgical wounds or compromise of the mucocutaneous barrier.9 Uncontrolled diabetes is documented as the most prominent risk factor, accounting for 36% of total infections.10 These risk factors, caused by increased blood glucose and iron levels along with neutropenia, create a microenvironment that is favorable for the growth of mucorales.8
Disease Proliferation
Mucormycosis grows and spreads via two primary mechanisms: soft tissue invasion and angioinvasion.2,3,6,11 These two processes create a devastating effect on local tissue as the mucor invades the lumen of blood vessels and adheres to the internal elastic lamina.2,3,6,11 Its broad, nonseptate hyphae block the lumen and interrupt perfusion, causing thrombosis, infarction and rapid tissue necrosis accelerated by various fungal proteases, lipases and mycotoxins.2,3,6,11 Histopathological examination of specimens and neuroimaging recently established a third mechanism, perineural invasion.11 It is thought that this mechanism accounts for the spread of infection that can occur further away from the primary site of infection, such as through the trigeminal ganglion.11 The most common site of mucormycosis infection is in the sinuses, accounting for 39% of all mucormycosis infections (while pulmonary and cutaneous infections account for 24% and 19%, respectively).2,11 In ROCM, the infection typically starts in the nasal or maxillary sinus and spreads to the sphenoid or ethmoid sinus. From there, it can invade the orbit, either through the ethmoid foramina or nasolacrimal duct or via dehiscence of the lamina papyracea.2,3,11 Upon invasion, the fungus can affect the medial rectus muscle, optic nerve and orbital apex structures, causing ocular discomfort and acute conjunctival chemosis and eventually proptosis, ophthalmoplegia and diplopia.2-4,6,7,12,13 Due to the angioinvasive properties of the fungus, it can also invade the central retinal artery or ophthalmic artery, possibly leading to a central retinal artery occlusion (CRAO) or ophthalmic artery occlusions (OAO).3
Once inside the orbit, the infection can also spread to the brain via the orbital apex or orbital veins, which drain into the cavernous sinus and can cause cavernous sinus thrombosis, carotid cavernous sinus fistula, local multiple occlusive strokes or carotid artery thrombosis.7 Unfortunately, once brain involvement occurs, the death of the patient is almost invariably the result.13
Identification
Initial suspicion of fungal infection may be aroused via a case of orbital cellulitis that does not respond to conventional antimicrobial therapy or the presence of orbital apex syndrome (proptosis in the case of ophthalmoplegia) with associated sinus involvement.4 Roughly 20% to 40% of patients show signs of a necrotic eschar if the initial infection involves the external nasal or maxillary sinuses.2 Presence of major risk factors, such as diabetes and immunosuppression, should raise the index of suspicion considerably, though it should be known that mucormycosis can also occur in the absence of underlying conditions.10
Neuroimaging via magnetic resonance imaging (MRI) or computed axial tomography (CT) with contrast can aid in diagnosis by revealing an invasion of the sinuses; however, definitive diagnosis is only achieved via histopathological study.2-4,6,11 Positive analysis typically reveals large, nonseptate hyphae branching at right angles.2,3,6,11
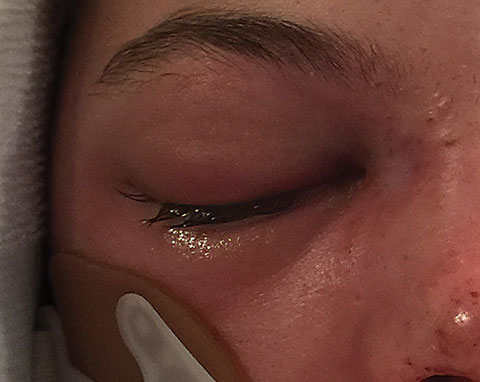 |
| Our patient displays external orbital chemises. |
The differential diagnosis for mucormycosis should include other infectious etiologies, such as bacterial orbital cellulitis, MRSA orbital cellulitis and orbital aspergillosis, as well as certain inflammatory and neoplastic conditions such as orbital pseudotumor, mucocele invasion, thyroid eye disease and orbital or lacrimal gland tumors, any of which may present acutely. Most of these can be ruled out with case history or clinical examination, but some may depend on histopathological diagnosis for differentiation.15
Neuroimaging is also helpful to determine the extent of invasion and to provide clues as to the nature of the lesion.15,16 CT scanning, though sensitive to determining the extent of invasion and the presence of abscesses, usually gives nonspecific information regarding the type and nature of the lesion. Both T1 or T2 weighted MRI images and diffusion-weighted imaging are more helpful in showing the nature of orbital lesions.16,17
Finding the Culprit
Bacterial infections comprise the vast majority of orbital cellulitis in adults with 60% to 80% arising from spread of paranasal sinusitis.18-20 The most common causative organisms are Staphylococcus aureus, Streptococcus and Haemophilus influenzae type B.19 A lack of response to conventional therapy warrants further work-up including orbital biopsy to rule out fungal or MRSA infections, inflammatory masses or neoplasms.19 Local rates of MRSA infections and antibiotic response profiles should be obtained in consultation with infectious disease. MRSA orbital cellulitis has an atypical presentation, and frequently lacks any of upper respiratory illness, paranasal sinusitis or eyelid trauma which typically precede bacterial infections of the orbit.21 Aspergillosis, like other infectious etiologies, can invade along a rhino-orbital-cerebral pathway but usually lacks the aggressive clinical course of mucormycosis.22,23
Non-infectious conditions that can mimic mucormycosis include orbital pseudotumor, mucocele invasion, thyroid eye disease and even neoplasms and vascular masses (capillary hemangioma, orbital varix) though these are typically ruled out in the case of an acute presentation.15 Orbital pseudotumor is an idiopathic inflammatory disorder of the orbit and can present with acute pain, ptosis, proptosis, ophthalmoplegia and vision loss.24 It is typically found in the absence of sinusitis and is sometimes associated with chronic systemic inflammation, such as autoimmune disease.24 Though neuroimaging is helpful, an orbital biopsy is needed to confirm or rule out this diagnosis.24
Mucocele, although slow growing in nature, can have an acute onset of symptoms with the patient exhibiting exophthalmos, ptosis, diplopia and pain. However, 60% to 89% of mucoceles arise from the frontal sinuses, an unusual site for mucor involvement, according to investigators.25 Thyroid eye disease can also present with pain, diplopia, chemosis, and pressure sensation behind the eyes. However, it typically is bilateral and causes eyelid retraction in contradistinction to ptosis.15,26 Lacrimal gland neoplasms also present with ptosis, exophthalmos, and diplopia but can be ruled out with imaging and biopsy.15
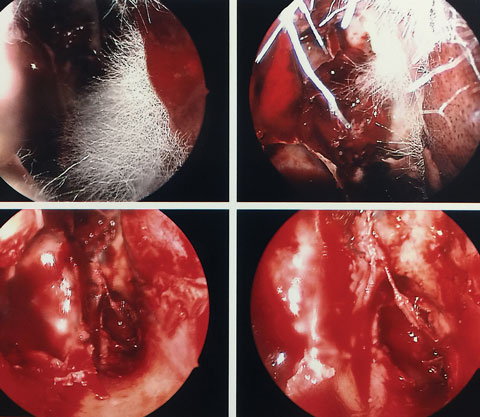 |
| These nasal sinus endoscopy images show mucorales hyphae. Click image to enlarge. |
Treating the Patient
Timely diagnosis is critical due to the rapid progression and potentially fatal complications of the disease. Though some variation in the pace of progression exists, a one-week window between orbital involvement in which the condition can be diagnosed and CNS involvement in which it proves fatal is fairly typical.2,13
Conventional treatments consist of a combination of IV amphotericin B (3mg/kg to 5mg/kg administered daily) and surgical resection of the infected tissues.3-7,10 Different combinations of these approaches can be used, including repeated debridement of necrotic tissue, irrigation of resected tissue with amphotericin B and application of amphotericin B-soaked gauze to areas of surgical removal.2,7,14 Note: renal function should be monitored in patients taking amphotericin B due to its nephrotoxicity; for this reason, liposomal amphotericin B is sometimes used in place as a first-line treatment due to its lower incidence of nephrotoxicity.2,4
Hyperbaric oxygen therapy has also been incorporated to treat mucormycosis due to the fungicidal and angiogenic effects of oxygen.2,4 Oral posaconazole can be used as an alternative treatment if nephrotoxicity or amphotericin B resistance develops.2,4 Lastly, due to its multi-system involvement, management of ROCM should occur in a tertiary interdisciplinary setting with practitioners from intensive care; ear, nose and throat; ophthalmology; infectious disease; internal medicine and neurosurgery all playing a role in patient management.2
Prognosis is directly related to how early diagnosis is made and treatment is initiated. Patients can expect full recovery of ocular motility and lid function within 10 months with successful treatment and eradication of the infection; however, visual loss due to CRAO/OAO or optic nerve compression is permanent.4 Additionally, even with a prompt diagnosis and aggressive treatment, mortality rates for the condition remain between 30% to 35%.2,10
Mucormycosis can mimic common bacterial infections of the sinuses and orbit until its advanced stages—a dangerous trait, as resulting delays in diagnosis and treatment can mean catastrophic outcomes.
Orbital cellulitis that does not improve with antimicrobial therapy or orbital apex syndrome with sinus involvement should undergo investigation for mucormycosis, especially when the patient exhibits primary risk factors including uncontrolled diabetes, ketoacidosis or immunosuppression.2,4,6
Despite heroic measures of early diagnosis and proper treatment, our patient ultimately required an orbital exteneration and eventually a hemiface-ectomy. Currently, the patient remains in a complicated long-term recovery situation.
Nicholas Karbach is an OD candidate at the of Pennsylvania College of Optometry at Salus University. He will begin his primary care/ocular disease residency at The Eye Institute in July. His interests include primary care optometry and neuro-ophthalmic disease.
Dr. Gurwood is a professor at Salus University.
|
1. Bouza E, Munoz P, Guinea J. Mucormycosis: an emerging disease? Clin Micorbiol Infect. 2006;12:7-23. 2. Martin-Moro JG, Calleja JML, Garcia MB, et al. Rhinoorbitocerebral mucormycosis: A case report and literature review. Med Oral Patol Oral Cir Bucal. 2008;13:E792-5. 3. Teixeira CA, Medeiros PB, Leushner P, Almeida F. Rhinocerebral mucormycosis: literature review apropos of a rare entity. BMJ Case Reports. 2013 Feb 5;2013. 4. Anders U, Taylor E, Martel J, Martel J. Acute orbital apex syndrome and rhino-orbito-cerebral mucormycosis. International Medical Case Reports Journal. 2015;8:93-6. 5. Eucker J, Sezer O, Graf B, Possinger K. Mucormycoses. Mycoses. 2001;44:253–60. 6. Shatriah I, Mohd-Amin N, Tuan-Jaafar TN, et al. Rhino-orbito-cerebral mucormycosis in an immunocompetent patient: case report and review of literature. Middle East African Journal of Ophthalmology. 2012;19(2):258-61. 7. Gamaletsou MN, Sipsas NV, Roilides E, Walsh TJ. Rhino-orbital-cerebral mucormycosis. Current Infectious Disease Reports. 2012;14(4):423-34. 8. Pinto ME, Manrique HA, Guevara X, et al. Hyperglycemic hyperosmolar state and rhino-orbital mucormycosis. Diabetic Research and Clinical Practice. 2010;91(2):e37-e39. 9. Ribes J, Vanover-Sams C, Baker D. Zygomycetes in human disease. Clinical Microbiology Reviews. 2000;13(2): 236–301. 10. Roden M, Zaoutis T, Buchanan W, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634-53. 11. Sravani T, Uppin SG, Uppin MS, Sundaram C. Rhinocerebral mucormycosis: Pathology revisited with emphasis on perineural spread. Neurol India. 2014;62(4):383-386. 12. Ochiai H, Iseda T, Miyahara S, et al. Rhinocerebral mucormycosis - case report. Neurol Med Chir. 1993;33:373-6. 13. Thurtell MJ, Chiu AL, Goold LA, et al. Neuro-ophthalmology of invasive fungal sinusitis: 14 consecutive patients and a review of the literature. Clin Experiment Ophthalmol. 2013;41(6):567-76. 14. Joos Z, Patel B. Intraorbital irrigation of amphotericin B in the treatment of rhino-orbital mucormycosis. Ophthal Plast Reconstr Surg. 2017;33(1):e13-6. 15. Grech R, Cornish K, Galvin P, et al. Imaging of adult ocular and orbital pathology—a pictorial review. Journal of Radiology Case Reports. 2014;8(2):1-29. 16. Kapur R, Sepahdari AR, Mafee MF, et al. MR imaging of orbital inflammatory syndrome, orbital cellulitis and orbital lymphoid lesions: The role of diffusion-weighted imaging. AJNR American Journal of Neuroradiology. 2009;30(1):64-70. 17. Lee S, Yen MT. Management of preseptal and orbital cellulitis. Saudi Journal of Ophthalmology. 2011;25(1):21-9. 18. Papier A, Tuttle DJ, Mahar TJ. Differential diagnosis of the swollen red eyelid. American Family Physician. 2007;76(12):1815-24. 19. Lam Choi VB, Yuen HKL, Biswas J, Yanoff M. Update in Pathological Diagnosis of Orbital Infections and Inflammations. Middle East African J Ophthalmol. 2011;18(4):268-76. 20. Pakdaman M, Sepahdari A, Elkhamary S. Orbital inflammatory disease: Pictorial review and differential diagnosis. World Journal of Radiology. 2014;6(4):106-15. 21. Mathias M, Horsley M, Mawn L, et al. Atypical presentations of orbital cellulitis caused by methicillin-resistant Staphylococcus aureus. Ophthalmology. 2012;119(6):1238-3. 22. Leyngold I, Olivi A, Ishii M, et al. Acute chiasmal abscess resulting from perineural extension of invasive sino-orbital aspergillosis in an immunocompetent patient. World Neurosurgery. 2014;81(1):203.e1-203.e6. 23. Arora V, Nagarkar NM, Dass A, Malhotra A. Invasive rhino-orbital aspergillosis. Indian Journal of Otolaryngology and Head & Neck Surgery. 2011;63(4):325-9. 24. Chaudhry IA, Shamsi FA, Arat YO, Riley FC. Orbital pseudotumor: distinct diagnostic features and management. Middle East African Journal of Ophthalmology. 2008;15(1):17-27. 25. Aggarwal S, Bhavana K, Keshri A, et al. Frontal sinus mucocele with orbital complications: Management by varied surgical approaches. Asian Journal of Neurosurgery. 2012;7(3):135-40. 26. Bahn RS. Graves’ Ophthalmopathy. The New England Journal of Medicine. 2010;362(8):726-38. |

