However, the increasing prevalence of the disease causes concern, and compliance barriers—long known as a significant hurdle in successful glaucoma therapy—have led to poor compliance with glaucoma medications.2 In 2013, the global prevalence of open-angle glaucoma (OAG) in people 40 to 80 years of age was 3.54%, affecting nearly 64.3 million people.3 This number is expected to rise to 76 million by 2020.4 With less than one-third of glaucoma patients considered “highly compliant,” blindness from the disease will inevitably increase and have devastating impacts on health care costs.5
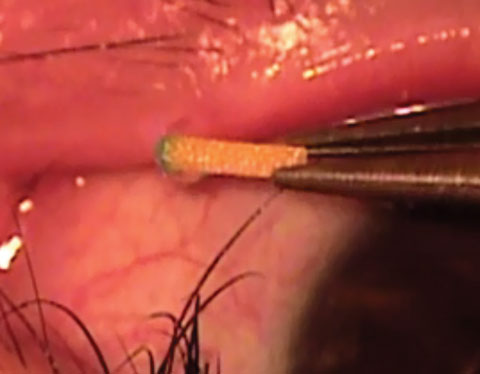 |
| Fig. 1. This intracanalicular punctal plug from Ocular Therapeutix delivers travoprost to the ocular surface for two to three months. Photo: Ocular Therapeutix |
However, patients who understand they have a blinding disease, trust their doctors and receive adequate reminders to take their medications often have improved compliance. If a sustained-release drug delivery system was available, these barriers would no longer exist—and fortunately, several are on the horizon.
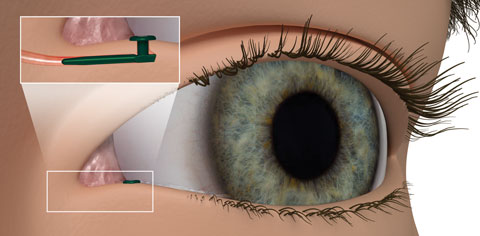 |
| Fig. 2. The Evolute punctal plug delivery system from Mati Therapeutics has been tested using latanoprost in patients diagnosed with OAG and ocular hypertension. Photo: Mati Therapeutics |
Ocular Drug Delivery
On the ocular surface, several biochemical factors play into how and when an adequate amount of a drug reaches its destination without causing toxicity to ocular tissues along the way. Ocular barriers in the anterior segment that affect drug delivery include static barriers such as the corneal epithelium, corneal stroma and blood-aqueous barrier and dynamic barriers such as conjunctival blood flow, lymph flow and tear drainage. Metabolic barriers also affect proper drug delivery to the ocular structures.7In order for a drug to demonstrate superior efficacy, a hydro- and lipophilic balance is essential. High ocular surface contact time is also an important aspect in topical formulations. Prodrugs, additives, pluronics, cyclodextrins and colloidal dosage forms have all been used to balance these factors in the drugs available today.7 Although many effective glaucoma drugs are readily available, their ability to lower IOP diminishes as compliance suffers.
Current Glaucoma Management
Today’s mainstream glaucoma therapies include topical medications, laser treatments and surgical procedures. The Ocular Hypertension Treatment Trial Study, Early Manifest Glaucoma Trial, Advanced Glaucoma Intervention Study and Glaucoma Laser Trial have all been influential in determining when and how we treat our patients to adequately lower IOP for the longest duration possible in the most effective sequence.3Topical medications, the primary approach for glaucoma management, have both advantages and disadvantages. They are minimally invasive, provide low absorption into systemic circulation, avoid first pass metabolism, are easy to administer and need a relatively small dose. However, they also have rapid drainage, interact with tear protein, are affected by tear turnover rate (1μL/min), and are rapidly removed due to reflex blinking and rapid metabolism.7
Selective laser trabeculoplasty (SLT) was a remarkable advancement that added an alternative for the treatment of certain glaucomas. FDA approved in 2001, SLT improved upon argon laser trabeculoplasty (ALT) and made laser treatments less damaging to ocular tissue.8 SLT has proven effective as a primary or adjunct treatment in OAG with minimal side effects and complications.9,10 However, SLT has some disadvantages. Many patients still need to remain on drops after treatment, the procedure often needs to be repeated and data on its long-term effects is lacking.
Surgical options have vastly improved with the introduction of minimally invasive glaucoma surgery (MIGS) into clinical practice. The iStent (Glaukos), CyPass (Alcon), Xen gel stent (Allergan) and Trabectome (NeoMedix) have provided glaucoma specialists with several surgical options that possess excellent safety profiles.11,12 The biggest benefit of surgery is that it often is very effective in lowering IOP.11,12 However, any ocular surgery has significant associated risks, and patients remain fearful to undergo these procedures.11,12
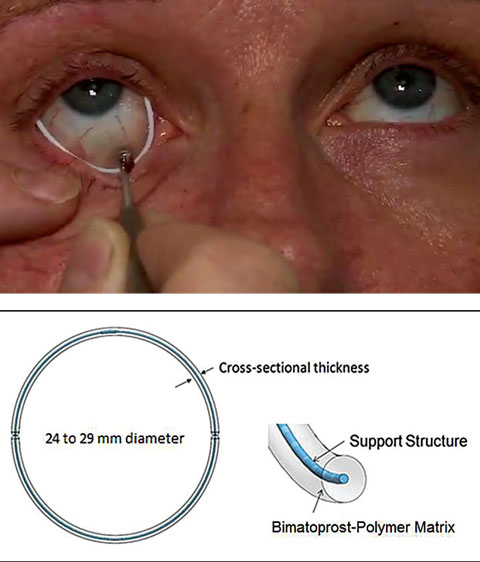 |
| Fig. 3. Allergan’s drug-eluting periocular ring is currently in Phase II clinical trials. Photo: Allergan |
Rise of Sustained-Release
Although the concept of a sustained-release device has been around in eye care since the 70s, none have become part of mainstream ophthalmic treatments thus far.7 Sustained-release medications are unique in that they would, in theory, target almost all non-compliance barriers.Several companies are working on new mechanisms for sustained-release medications for many ocular diseases, including glaucoma—some of which show strong promise of implementation in the near future. Devices that deliver medication from outside the eye include punctal plugs, gel-forming drops and drug-eluting devices. Those that deliver medication from inside the eye include injectables and implants.13 Here’s a look at what’s in the pipeline for these devices:
Outside the Eye
Punctal plugs. The OTX-TP (Ocular Therapeutix) delivers travoprost to the ocular surface via an intracanalicular punctal plug for two to three months, which then resorbs and drains through the nasolacrimal system (Figure 1). This plug is similar to a wine-cork design, allowing it to sit in the canaliculus without stressing the elasticity of the punctum.14 Its advantages include being minimally invasive, containing fluorescein for retention monitoring, allowing comfort for most patients and clears the body through absorption—all while being preservative free. Studies show the therapeutic benefit has been significant for 90 days with a consistent 90% retention rate.15,16 Phase II trials in South Africa and the United States, which suggest no serious adverse effects so far, shows a slightly less hypotensive effect when compared with timolol.15,16 Phase III clinical trials in patients with ocular hypertension and glaucoma are underway.15,16Mati Therapeutics has developed the Evolute (OR) punctal plug delivery system, which has been tested using latanoprost with OAG as well as with patients with ocular hypertension (Figure 2). The Evolute’s drug core allows unidirectional sustained drug elution into the tear film, thereby minimizing systemic absorption.17 The trials studied two different delivery doses and placement variations in the upper and lower puncta, both separately and simultaneously. So far, multiple multicenter US clinical trials indicate the lower punctum has retention rates up to 96% over a 12-week period.17,18
However, punctal plugs are foreign objects and have the potential to move, which may affect drug release and cause irritation or infection. Additionally, both companies are working to prove the intended drug is released at a constant rate for months at a time.
Contact lenses. A recent study shows latanoprost-eluting contact lenses are at least as effective as daily administration of latanoprost ophthalmic solution eye drops when tested in glaucomatous monkeys.19 This preclinical study was conducted with both low-dose and high-dose contact lenses via a thin latanoprost-polymer film inside a methafilcon hydrogel periphery lathed into a contact lens.19
Other drug-eluting devices. Allergan recently acquired a promising drug-eluting periocular product that rests on the surface of the eye just underneath the eyelids (Figure 3). The results of a controlled Phase II study show that a single administration of the ring using bimatroprost provided sustained reduction of IOP of 4mm Hg to 6mm Hg for six months at the study’s primary endpoint of 12 weeks. The retention rate was approximately 90% of subjects after six months.20
Although the company has plans for a six-month Phase III non-inferiority trial, this product is currently in its second multicenter, randomized, controlled Phase II clinical study to assess the insert over six months. A larger Phase II randomized, controlled trial has been completed.16,21
Amorphex Therapeutics’ topical ophthalmic drug delivery device (TODDD) is made of a biocompatible soft elastomeric material that rests on the sclera underneath the eyelid (Figure 4). It can incorporate several different drops separately or simultaneously, including timolol maleate, prostaglandins, pilocarpine, brimonidine and even some anti-inflammatories and antibiotics.22 The TODDD contains a large capacity for the delivery of medication for several months.22 Obvious advantages include the improved efficiency and easy insertion and removal. The placement and replacement is fairly simple, takes less than a minute and can be done by patients at home.
The TODDD’s Phase IIa trials demonstrate uninterrupted therapeutic efficacy for 180 days in humans with safety, comfort and strong retention. It has also shown reduced IOP of more than 35% in a prostaglandin study using dogs. Amorphex has secured a regulatory path with the FDA to gain approval.22,23
Although nanoparticle crosslinked collagen shields are still in development, a new study shows a pilocarpine sustained-release delivery using a nanoparticle shield was a safe and promising option in formulating a sustained-release treatment.24
Gel-forming drops. These use pentablock copolymers as a vehicle for topical and intraocular drug delivery. Five polymer blocks are currently FDA approved for use in the eye. In the topical version, the intended drug is added to a non-viscous polymer drop. After touching the eye, this drop reacts with body temperature and transforms into a gel, which then lies under the eyelid, forming a film that releases the drug slowly until the polymer naturally degrades. Depending on the dose, the time course can be two, four or even six months.
DuraSite ISV-215 (InSite Vision) uses pentablock copolymers to form a stable mucoadhesive matrix of bimatoprost 0.03% that provides prolonged contact with the cornea, conjunctiva and other ocular tissues. DuraSite ISV-215 significantly improved the delivery of bimatoprost to rabbit eyes compared with Lumigan (bimatoprost 0.03% ophthalmic solution, Allergan).25
However, pentablock copolymers are subject to a “burst effect” in which 30% to 40% of the drug may release quickly on initial placement. So far, studies indicate DuraSite ISV-215 is resilient.26
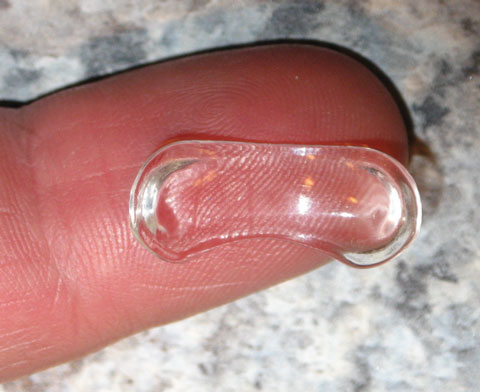 |
| Fig. 4. This topical ophthalmic drug delivery device (Amorphex Therapeutics) rests on the sclera, providing sustained drug delivery for several different drops separately or simultaneously. Photo: Amorphex Therapeutics |
Inside the Eye
Injectables. Allergan’s bimatoprost sustained-release implant, which is injected into the anterior chamber, is currently undergoing Phase III clinical trials. In Phase II trials, it lowered IOP in 92% of patients at four months and 71% at six months. So far, no serious adverse ocular events have occurred during the trials.27ENV515 (Envisia) is also an anterior chamber injection, for which an extended-release formulation of travoprost is used in a biodegradable polymer drug delivery system. In Phase I clinical trials, IOP was reduced an average of 35% (6.4 +/- 0.6mm Hg) over eight months and then returned to baseline at nine months.28 In Phase IIa clinical trials, IOP was reduced 28% (6.7mm Hg) at day 25, which was comparable with once-daily Travatan Z (travoprost ophthalmic solution, Alcon) in the other eye.28,29,30 A Phase II trial shows that a single administration can result in a clinically meaningful decrease in IOP for the entire nine-month period. The only adverse effect noted in the trial was transient hyperemia.30
GrayBug is in the preclinical phase of developing an implant with a microparticle controlled-release drug delivery system using technology from the Wilmer Eye Institute of Johns Hopkins School of Medicine in Baltimore. Three agents are under investigation: a single hypotensive compound, a dual action hypotensive compound and a hypotensive compound combined with neuroprotective properties. Testing in animal models showed successful decrease in IOP with subconjunctival administration. These initial studies showed no inflammation after six months, and the implant resorbed completely after six months.16
Clearside Biomedical and Santen are collaborating on supraciliary drug delivery in glaucoma via Clearside’s microinjector and Santen’s sustained-release formulations.31 Injection into the supraciliary space puts the drug in close proximity to the ciliary body—the targeted tissue of most glaucoma meds. Researchers tested sulprostone and brimonidine with the delivery system, and both decreased IOP at a significantly lower dosage than their topical counterparts.32,33 Brimonidine ophthalmic solution given as a topical formulation is 75ug three times per day, while the injected dose of brimonidine to the supraciliary space is roughly 100 times less than that. In the preclinical phase, a single administration of brimonidine injected into rabbit eyes reduced IOP initially by 6mm Hg; unfortunately, the effect slowly tapered off over a month.32,33
Currently in the preclinical trial phase, Ohr Pharmaceuticals has developed an injectable nanoparticle device to administer latanoprost in, on or around any portion of the eye for glaucoma, specifically steroid-induced glaucoma. Researchers hope nanotechnology can offer advantages to ocular drug delivery with improved consistency without corneal penetration. Additionally, adnexal adverse effects do not occur with this device. Some disadvantages include risk of toxicity, damage to intraocular structures and the need for lifelong procedures to ensure sustainability of decreasing IOP.16
Implants. Icon Bioscience is developing a glaucoma device, IBI-60089, which is currently in preclinical trials. The goal is for a single injection of latanoprost to have a hypotensive effect for at least six months. Researchers are combining the drug with a carrier platform called Verisome into a true liquid injection. It degrades and eventually disappears as the active agent is released over time. The most evolved product at this time is called IBI-10090, which is meant for uveitis and postsurgical inflammation and is currently in Phase III clinical trials.34
pSivida recently invented a device for glaucoma called Durasert (Figure 5). This is a bioerodible, sustained-release drug delivery subconjunctival implant roughly the size of a grain of rice (3mm to 4mm in length, 0.4mm in diameter) containing latanoprost in a tiny translucent cylindrical polymer tube. The device would not need to be surgically removed and may work for three to six months, according to the company. A Phase I/II clinical trial is underway with subjects diagnosed with ocular hypertension to determine the safety and efficacy of the implant.35
Replenish has developed a smart device to be implanted in the sclera. This micropump has a programmable system designed to dispense nanoliter-sized doses of drugs every hour, day or month for up to 12 months. The current studies show a good safety profile in dogs without excess inflammation or scarring at 12 months.14,36
 |
| Fig. 5. Durasert, develped by pSivida, is a bioerodible subconjunctival implant roughly the size of a grain of rice. Photo: pSivida |
Still Looking for Answers
Even if, or when, any of these devices become FDA approved, clinicians will still question whether patients will accept this change in glaucoma therapy. A review of available sustained-release systems in Singapore sheds some light on the possible adoption of such therapies, as patients preferred sustained-release devices over topical administration, with a special preference for punctal plugs. The same review also shows that 85% of patients were even willing to pay more for the sustained-release option.13Long-term comfort and use of each device will largely be responsible for whether or not sustained-release medications are integrated into mainstream glaucoma care. Researchers will also have to determine if and when additional topical medications or laser and surgical procedures will be recommended after sustained-release instillation.
Although sustained-release devices resting on the eye should already be within optometric scope of practice, we as a profession will have to come together to fight for the right to insert and remove devices that are injected or inserted into the eye.
Even with so many unanswered questions, eye care providers can look forward to the day many of these sustained-release devices enter their clinic. With the prevalence of glaucoma increasing worldwide, causing a tremendous strain on the health care system, the improved compliance they could create would be hugely beneficial. Without question, sustained-release devices have the potential to revolutionize glaucoma care in the near future.
Dr. Kresch is an instructor in Optometric Sciences at the Columbia University Medical Center and is the pioneering optometrist in the glaucoma clinic and the general ophthalmology clinic of New York Presbyterian Hospital. Dr. Kresch is also the founder of a patient education website, www.eyeinquire.com.
|
1. Hylton C, Robin AL. Update on prostaglandin analogs. Curr Opin Ophthalmol. 2003;14:65-9. 2. Lusthaus J, Goldberg I. Emerging drugs to treat glaucoma: Targeting prostaglandin F and E receptors. Expert Opinion on Emerging Drugs. 2016;21(1):117-28. 3. Cheema A, Chang RT, Shrivastava A, Singh K. Update on the medical treatment of primary open-angle glaucoma. Asia-Pacific J Ophthalmol. 2016;5(1):51-8. 4. Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081-90. 5. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthlamol. 2002;120:701-13. 6. Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistence Study. Ophthalmology. 2008;8:1320-27. 7. Cholkar K, Patel S, Vadlapudi A, Mitra A. Novel strategies for anterior segment ocular drug delivery. J Ocular Pharmacology and Therapeutics. 2012;29(2):106-23. 8. Francis BA, Loewen N, Hong B, et al. Repeatability of selective laser trabeculoplasty for open-angle glaucoma. BMC Ophthalmology. 2016;16(1). 9. McIlraith I, Strasfeld M, Colev G, Hutnik CM. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma. 2006;15:124-30. 10. Katz LJ, Steinmann WC, Kabir A, et al, SLT/Med Study Group. Comparison of selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma. 2012;21:460-8. 11. Francis BA, Minckler D, Dustin L, et al. Combined cataract extraction and trabeculectomy by the internal approach for coexisting cataract and open-angle glaucoma: initial results. J Cataract Refract Surg. 2008;34:1096-1103. 12. Belovay GW, Naqi A, Chan BJ, et al. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(1):1911-7. 13. Chan HH, Wong TT, Lamoureux E, et al. A survey on the preference of sustained glaucoma drug delivery systems by Singaporean Chinese patients: a comparison between subconjunctival, intracameral, and punctal plug routes. J Glaucoma. 2015;24:485-92. 14. Bethke W. New frontiers in sustained release. Review of Ophthalmology. June 8, 2015. Available at www.reviewofophthalmology.com/article/new-frontiers-in-sustained-release. Accessed November 30, 2016. 15. Ocular Therapeutix. Sustained Release Travoprost. Available at www.ocutx.com/pipeline/travoprost-punctum-plug. Accessed November 30, 2016. 16. Gebhart F. Drug-delivery platforms offer more options and promise for clinicians. Ophthalmology Times. Available at http://ophthalmologytimes.modernmedicine.com/ophthalmologytimes/news/drug-delivery-platforms-offer-more-options-and-promise-clinicians. Accessed November 30, 2016. 17. Mati Therapeutics. Mati Therapeutics selected to present at Ocular Innovation Summit: Pursuing sustained ocular drug delivery programs in inflammation, pain, and glaucoma. October 3, 2016. Available at www.matitherapeutics.com/about/press/ocular-innovation-summit. Accessed November 30, 2016. 18. Mati Therapeutics. Mati Therapeutics announces initiation of phase II study to compare latanoprost punctal plug delivery system (L-PPDS) to timolol eye drops. October 28, 2013. Available at www.matitherapeutics.com/about/press/compare-latanoprost-to-timolol. Accessed November 30, 2016. 19. Ciolino JB, Ross AE, Tulsan R, et al. Latanoprost-eluting contact lenses in glaucomatous monkeys. Ophthalmology. 2016;123:2085-92. 20. Brandt JD, Sall K, DuBiner HB, et al. 6-month IOP-reduction with a single dose of a novel topical bimatroprost ocular insert: a phase 2 randomized, double-masked, controlled study. Paper presented at the AAO Annual Meeting, November 17, 2015; Las Vegas, NV. 21. ForSight VISION 5. The Bimatoprost Ring. Available at http://Forsightvision5.com. Accessed November 30, 2016. 22. Amorphex Therapeutics LLC. Product development. Available at https://amorphextherapeutics.wordpress.com/our-technology. Accessed November 30, 2016. 23. Hughes P, Neervannan S. Ophthalmic drug development and the elderly. In: AAPS Advances in the Pharmaceutical Sciences Series. Springer Nature; 2016:383–401. 24. Agban Y, Lian J, Prabakar S, et al. Nanoparticle cross-linked collagen shields for sustained delivery of pilocarpine hydrochloride. International Journal of Pharmaceutics. 2016;501:96-101. 25. Bowman L, Shafiee S, Hou E, Hosseini K. Ocular pharmacokinetics of ISV-215 (bimatoprost 0.03%) formulated in a Durasite delivery system compared to Lumigan in rabbits. Invest Ophthalmol Vis Sci. 2013;54:765. 26. Karmel M, Iwach AG, Mitra AK, Novack GD. Novel approaches to drug delivery. American Academy of Ophthalmology. January 2013. Available at www.aao.org/eyenet/article/novel-approaches-to-drug-delivery-2. Accessed November 30, 2016. 27. Allergan. Positive phase I/II interim data of bimatoprost sustained-release implant for IOP therapy in glaucoma. November 16, 2015. Available at http://bit.ly/1QhSGfr. Accessed November 28, 2016. 28. Navratil T, Garcia A, Tully J, et al. Preclinical evaluation of ENV515 (travoprost) intracemeral implant-clinical candidate for treatment of glaucoma targeting six-month duration of action. Paper presented at ARVO, May 6, 2014; Orlando, FL. 29. Envisia Therapeutics. Envisia Therapeutics’ lead product candidate, ENV515 (travoprost XR), achieves primary efficacy endpoint in phase 2a glaucoma clinical trial. October 6, 2015. Available at http://prn.to/1NljZBr. Accessed November 28, 2016. 30. Envisia Therapeutics. Envisia therapeutics releases ENV515 (travoprost XR) phase 2 data showing nine-month duration of action after a single dose in patients with glaucoma. Available at www.envisiatherapeutics.com/wp-content/uploads/2016/10/ENV515-Cohort-2-9-Month-Data-Release-FINAL4.pdf. October 17, 2016. Accessed November 30, 2016. 31. Clearside Biomedical. Clearside Biomedical, Inc. and Santen, Inc. announce research collaboration in glaucoma. Business Wire. May 4, 2015. Available at www.businesswire.com/news/home/20150504005229/en/Clearside-Biomedical-Santen-Announce-Research-Collaboration-Glaucoma. Accessed January 13, 2017. 32. Kim YC, Edelhauser HF, Prausnitz MR. Targeted delivery of antiglaucoma drugs to the supraciliary space using microneedles. Invest Ophthalmol Vis Sci. 2014;55(11):7387-97. 33. Chiang B, Kim Y, Doty A, et al. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. Journal of Controlled Release. 2016;228:48-57. 34. Tierney DS. Spotlight on drug delivery: Icon Bioscience. Presented at the Ophthalmology Innovation Summit at ASCRS 2015, November 12, 2015. Available at http://ois.net/icon-bioscience. Accessed November 30, 2016. 35. pSivida. Durasert/latanoprost. Available at www.psivida.com/products-durasert.html. Accessed November 30, 2016. 36. Gutiérrez-Hernández JC, Caffey S, Abdallah W, et al. One-year feasibility study of Replenish MicroPump for intravitreal drug delivery: a pilot study. Transl Vis Sci Technol. 2014;3(4):8.1. |

