 |
||
|
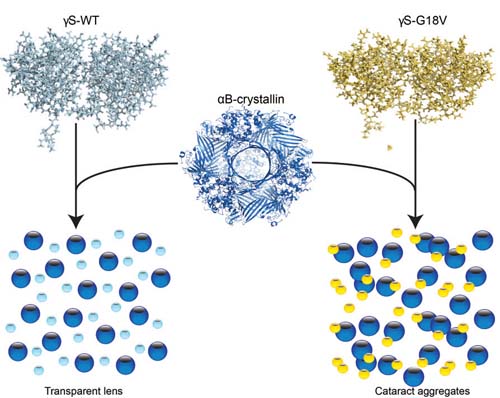
The lens stays clear thanks to the "chaperone" protein alpha B-crystallin, which binds to gamma S-crystallin (above right) to prevent a cataract from forming.
|
||
Researchers from University of California Irvine and the Leibniz Institute for Molecular Pharmacology in Germany identified the structures of normal proteins in the human eye and a genetic mutation known to cause cataracts in children. According to their study, the “chaperone” protein in the eye—which prevents the other two structural proteins from clumping into cataracts—binds far more strongly to mutated or damaged proteins in an effort to keep the lens clear.
Unfortunately, the human eye is equipped with only a finite number of these chaperone proteins, called αB-crystallins. When they’re tapped out, the now-weakened eye proteins are able to aggregate, which forms a cataract.
However, “understanding the molecular mechanism of what goes wrong in the eye that leads to a cataract could lead to the development of better treatment options, including more sophisticated artificial lenses and drugs,” says the study’s co-author Rachel Martin, PhD, associate professor of chemistry at UC Irvine.
For instance, the researchers are hopeful that organic chemists can use their findings to create sight-saving treatments to prevent initial aggregation.
Kingsley CN, Brubaker WD, Markovic S, et al. Preferential and specific binding of human alpha b-crystallin to a cataract-related variant of gamma s-crystallin. Structure. 2013 Dec 3;21(12):2221-7.

