 A 56-year-old Middle Eastern male presented with sudden-onset visual blur in his left eye that had persisted for one week. His ocular history was significant for cataract surgery OU and bilateral retinal detachments, which were repaired via laser therapy several years earlier. He reported maintaining excellent vision following detachment repair. Indeed, his visual acuity measured 20/20 OU at his most recent eye examination a year earlier.
A 56-year-old Middle Eastern male presented with sudden-onset visual blur in his left eye that had persisted for one week. His ocular history was significant for cataract surgery OU and bilateral retinal detachments, which were repaired via laser therapy several years earlier. He reported maintaining excellent vision following detachment repair. Indeed, his visual acuity measured 20/20 OU at his most recent eye examination a year earlier.
His medical history was significant for medically controlled hypertension; however, he didn’t remember the name of his hypotensive agent.
On examination, his best-corrected visual acuity measured 20/20 OD and 20/100 OS. Extraocular motility testing was normal. Confrontation visual fields were full to careful finger counting OU. His right pupil was round and reactive, measuring 5mm in diameter. But, the left pupil was irregularly shaped, minimally reactive and was approximately 7mm in diameter. There was no evidence of afferent pupillary defect OU.
| |
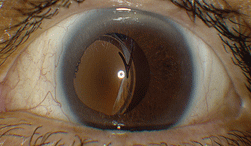
| 1. Slit lamp photograph of our patient’s left eye revealed an obvious complication. |
The anterior segment examination of the right eye revealed a well-centered posterior chamber IOL implant. The anterior segment evaluation of the left eye, however, uncovered a pronounced complication (figure 1).
Dilated fundus examination of the right eye was unremarkable. We documented no evidence of a previous retinal detachment. No scleral buckle or buckle contours were noted. Further, there were no peripheral laser scars OD.
Fundus evaluation of the left eye showed significant changes (figure 2). So, we ordered a spectral-domain optical coherence tomography (SD-OCT) scan of his left eye (figure 3).
Take the Retina Quiz
1. How do you explain the sudden change in his visual acuity?
a. Full-thickness macular hole.
b. Shallow, macula-off retinal detachment.
c. Dislocated IOL implant.
d. Epiretinal membrane (ERM).
2. What other significant retinal changes can be seen in the fundus photo?
a. Retinal hole.
b. Full-thickness macular hole.
c. Atrophic retinal hole.
d. Retinal detachment.
3. How should this patient be managed?
a. Observation.
b. Pars plana vitrectomy (PPV), membrane peel and gas injection.
c. PPV, endolaser and iris-fixated IOL repositioning.
d. Laser photocoagulation.
4. What is his prognosis for visual recovery?
a. Good.
b. Poor.
c. Guarded.
d. Impossible to determine.
Discussion
Our patient presented with a dislocated IOL in his left eye, which explains his sudden change in visual acuity. It appears to have occurred spontaneously, because he reported no history of trauma or other precipitating events. The unexplained IOL dislocation was just the tip of the iceberg, however, as the rest of his ocular history is just as unclear.
He described undergoing bilateral cataract removal in the late 1990s, which means that he would have been in his early- to mid-40s at the time of surgery. This is atypical, considering most people develop cataracts at a much older age.
He then noted a history of bilateral retinal detachments, which were repaired with laser photocoagulation. Oddly, however, we documented no evidence of laser therapy––or any other retinal procedure––on his fundus examination. Needless to say, we were a bit puzzled by this case and could not uncover any definitive clues.
|
|
|
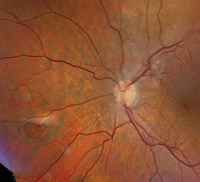
2. Fundus image of his left posterior pole. What do you see?
|
So, what exactly precipitated his IOL dislocation? Perhaps trauma at a young age, or he over-aggressively rubbed his eye while sleeping? Maybe he had preexisting pseudoexfoliation that weakened the zonules and predisposed him to postoperative IOL complications? We carefully evaluated the patient for pseudoexfoliation syndrome, but found no confirmatory evidence. (Admittedly, pseudoexfoliation can be much more difficult to detect following cataract surgery.)
The other interesting aspect of his exam is seen in the left fundus. There was a large retinal hole located approximately three disc diameters nasally in the midperiphery. We saw a cuff of fluid on the hole’s anterior side, and retinal pigment epithelium (RPE) hyperplasia on the optic nerve side. Did the RPE hyperplasia result from the laser treatment he reportedly received several years earlier? One of the secondary consequences of laser photocoagulation is scarring, which in some instances can cause retinal traction. But, in this case, such a small amount of hyperplasia makes this scenario unlikely.
However, that’s not the only place we documented traction. We also observed an ERM located above the optic nerve that involves a branch of the central retinal artery and vein, and extends into the macula.
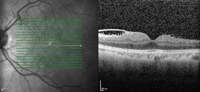
Although the fovea does not appear to be directly affected, the SD-OCT scan showed evidence of some macular involvement. Expansion and contraction of this ERM could have resulted in excessive traction––especially in areas with small amounts of pigment. We believe that this process ultimately led to the development of his retinal hole.
The final question: Are the ERM and retinal hole related to the dislocated IOL, or are they merely coincidental findings? This is a much more difficult question to answer.
A vitreoretinal specialist saw our patient the same day as his examination at our office. He was scheduled for a PPV and IOL repositioning. During the surgery, endolaser photocoagulation was applied to the retinal break. While the procedure was uneventful, his postoperative outcome was fraught with complications.
| |
|
3. An SD-OCT scan of his left macula reveals further changes.
|
He did well during the first several weeks post-op, but began experiencing discomfort and blurry vision during the second month. He then developed elevated intraocular pressure and corneal edema. After a few more weeks, we determined that he had epithelial ingrowth.
Epithelial ingrowth is the presence of corneal epithelial cells in an area where they do not belong. It is a rare complication of ophthalmic surgery or penetrating ocular trauma. It is seen most commonly following cataract surgery, but also may occur secondary to penetrating keratoplasty, trabeculectomy and glaucoma drainage implantation.1
The condition typically manifests as a sheet of epithelial tissue that grows onto the endothelium and/or the iris surface, and into the anterior chamber angle. Once this occurs, patients can develop corneal edema and angle-closure glaucoma.
Indeed, our patient’s IOP increased to more than 50mm Hg OS. He subsequently underwent glaucoma drainage implant (GDI) surgery, which brought his IOP under control. On his follow-up visit two months after the GDI procedure, his IOP was 20mm Hg, and his uncorrected visual acuity measured 20/40 OS. We will continue to follow him in three- to six-month intervals, depending upon his pressure levels.
Answers: 1) c; 2) a; 3) c; 4) a.
1. Seth RK. Epithelial Downgrowth: A new option for an old adversary. Eyenet. May 2008. Available at: www.aao.org/publications/eyenet/200805/pearls.cfm. Accessed July 9, 2014.

