 |
In daily practice, we tend to focus more on clinical signs and symptoms than pathophysiology, especially in corneal abrasion cases—the patient is in pain, infection risk is high and time is tight. The way we treat these patients prioritizes pain relief over a more holistic approach to the cornea’s status and risk profile. But gaining a greater awareness of how such conditions progress can help inform our treatment choices. This month we do so by looking more deeply at the mechanisms of corneal trauma and wound repair.
Why the Cornea is Unique
The cornea contains the highest concentration of sensory nerves and nociceptors in the body, making pain management a primary concern.1 Because the cornea is an avascular and immune-privileged site, wound repair involves several unique mechanisms, including epithelial cell proliferation, differentiation, migration and adhesion to the basement membrane.2,3
An intact corneal epithelium plays a vital role as a physical and mechanical barrier against infection, as well as in sustaining the necessary biochemical properties required for optical clarity.4 Several mechanisms can compromise an intact cornea and manifest clinically as abrasions, dystrophies or degenerations, as well as refractive problems.
The corneal epithelium is made up primarily of three distinct cell layers: an outermost layer of squamous cells, a wing cell middle layer and finally the basal cells. In corneal abrasion, the basal layer—the only epithelial component capable of regeneration—begins to proliferate. Basal cells migrate and spread over the wounded lesion, and then differentiate into wing cells, which then differentiate further into the squamous cells that comprise the superficial corneal epithelium.5
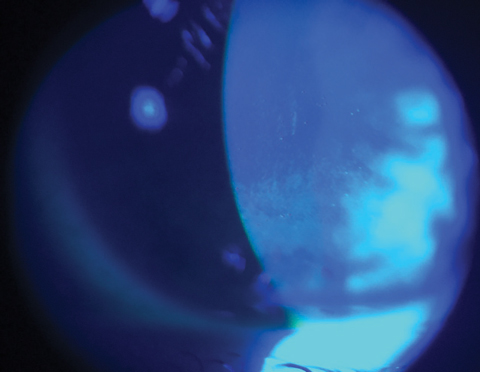 |
| Fluorescein stain under a cobalt blue filter depicts a diffuse breakdown of an otherwise intact corneal epithelium. |
Keys to Corneal Wound Repair
Following injury, which appears clinically as a defect in the corneal epithelial surface that stains with fluorescein dye, several processes bring the cornea back to its normal state.
Epithelial cell migration. In the early stages of wound healing, migration and proliferation of epithelial cells are necessary for closure. The process of epithelial cell migration is highly metabolic, depending heavily on glucose from the aqueous humor, and occurs in two ways. First, transient amplifying cells (TAC) travel centrally to the abrasion site within the basal layer, where they then differentiate into the post-mitotic cells that comprise the more superficial epithelial layers. Concurrently, epithelial cells also migrate peripherally around the limbus until the wound is fully closed.6 These migrating epithelial cells briefly express matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) enzymes, which regulate the adherence between the injured epithelium and underlying basement membrane.7 Fibronectin, an extracellular structural protein in the basement membrane that binds to cells and collagen, provides a provisional matrix with which epithelial cells can migrate. Along with collagen I and IV, fibronectin also increases cell motility and migration.8 Systemic diseases, such as diabetes, may delay these metabolic processes and prevent the proper adherence of the epithelium to the basement membrane.2
Keratocyte release of matrix proteins. Corneal abrasions induce an acute inflammatory response and also result in the death of underlying keratocytes.9,10 While diseases that cause chronic inflammation to the cornea are typically detrimental, it is the acute inflammation following abrasion that is necessary for the ocular system to initiate and complete the repair process, and, as such, treatment in this acute phase with a topical steroids may delay healing time.9 This response is characterized primarily by neutrophil recruitment and migration from the limbal vessels to the wounded area. The accumulation of neutrophils at the wound site is vital to the repair effort; studies that investigated the effects of reducing neutrophil concentrations to the injured area show a significant delay in wound closure occurs.11
Corneal keratocytes, found in the stroma, produce extracellular matrix components such as collagen, proteoglycans and crystallins—all essential in maintaining corneal structure and clarity. Studies have concluded that keratocytes are depleted for several years following corneal injury, which may lead to complications such as ectasia following refractive procedures.9
Platelet build-up. Accumulation of these cells at the limbus happens in concert with neutrophil migration; both are critical for successful corneal wound healing. During the first 24 hours following injury, platelet recruitment aids in epithelial cell division. Platelets attach to neutrophils and augment migration. Studies report that platelets also aid in efficient keratocyte recovery.9 Platelets reach copious levels around the limbal blood vessels approximately 12 hours following injury, a crucial time when many of the cellular repair processes are underway. Research also shows reducing platelet levels also reduces neutrophil accumulation, and vice-versa.12 Following the commencement of this stage, the size of the epithelial defect is noticeably diminished or, in some instances, completely closed.
Patches vs. Lenses
Since corneal healing mechanisms are unique compared to that of other ocular tissues, which carry a blood supply and lack immune privilege, the treatment approach initiated by the practitioner will “make or break it.” As our understanding of the cornea advances, so do our protocols. As a result, one historically popular therapy has been phased out and another has emerged as the staple modality.
Pressure patching. Once the mainstay of abrasion treatment, this protects the cornea from the shearing force of the eyelid secondary to blinking. Prior to patching, topical antibiotics and a cycloplegic are often instilled into the affected eye.
Today, however, pressure patching is somewhat controversial. It reduces the amount of oxygen reaching the cornea and raises its temperature, both of which increase the risk of microbial infection and may even delay healing. As such, exercise caution with this approach.11 Studies question its value, noting that it does not demonstrate a faster healing time than observation alone, or even additional pain alleviation. In fact, the patch itself was the main cause of pain in 48% of cases.12 The occlusive effect from patching also prevents the patient from functioning binocularly.11 Cases where pressure patching may be used include large abrasions in young children or special populations where the patient may risk rubbing their eye and worsen the injury.
Bandage contact lens. This option supersedes pressure patching in that it not only alleviates pain, but also promotes wound healing.11 The bandage lens also provides lubrication, which aids in healing by providing a smooth surface for the migration of cells, and mitigates pain by shielding the cornea from external forces such as blinking. Its barrier function also adds microbial protection.13 Lastly, a bandage contact lens allows antimicrobial drops to remain on the corneal surface for an extended period, extending the their duration of action.13
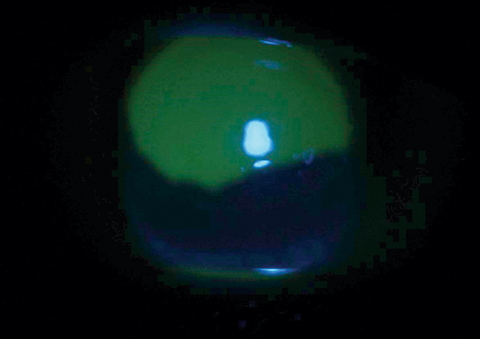 |
| This corneal abrasion with large epithelial defect is stained with fluorescein dye under cobalt blue light. |
Adjuvant Therapies
Though a bandage lens will be the workhorse therapy, other efforts that can accompany it include:
Artificial Tears. Topical lubricants, such as artificial tears or gels, help accelerate the healing process by smoothing out ocular surface abnormalities. The lubrication reduces friction from the patient’s eyelid and prevents desiccation.11
Topical NSAIDs. Systematic reviews conclude that topical NSAIDs are effective in reducing pain, as well as in reducing the need for oral analgesic therapy. Due to pain control, patients were able to return to work earlier without jeopardizing the healing process.12 Note that long-term use of topical NSAIDs may increase the risk of corneal melt and toxicity.5
Topical Antibiotics. Due to the risk of microbial superinfection from an open epithelial defect, topical antibiotics are frequently used as prophylaxis. Studies report a reduced risk of infection or ulceration with, particularly when antibiotic is implemented within the critical period of 12 to 18 hours following injury, a time where the many cellular processes discussed above are in play.12 Gel formulations of antibiotics offer additional lubrication as well. However, patients must be educated regarding the transient blurring effect due to viscosity. Topical antibiotic coverage should especially be considered in abrasions secondary to contact lenses, foreign body or vegetative matter, as there is an increased risk of infection. Antibiotic-steroid combination drops should be used with caution, as the steroidal component may suppress the necessary inflammatory processes and retard wound healing. These agents are typically used for 24 hours, or until full resolution of symptoms and signs.5
Clycloplegics. While commonly used for pain management because they minimize ciliary body spasm and reduce pain, the literature does not suggest a substantial benefit in uncomplicated corneal abrasions.14
Topical gel. Though unavailable in the US, a combination of xanthan gum, sodium hyaluronate and netilmicin may increase healing time and provide antimicrobial coverage.11 The hyaluronate incites epithelial migration; studies report adequate prophylaxis and faster resolution of abrasions.11 It is available in Europe under the trade name Xanternet.
Acacia honey. Honey’s antimicrobial, anti-inflammatory and antioxidative properties promotes healing on the skin surface.15 Epithelial cell migration was enhanced in a small, in vitro rabbit study, possibly because of its high sugar content, which is necessary for metabolic cell activity. Though it remains far from clinical deployment, its novel mechanism of action makes acacia worthy of future exploration.15
Corneal wound healing possible through a number of complex cellular processes. Informed treatment choices promote repair and prevent delay or complication.
1. Li Z, Burns AR, Han L, et al. IL-17 and VEGF are necessary for efficient corneal nerve regeneration. Am J Pathol. 2011;178(3):1106-16. |

