 |
The retinal pigmented epithelium (RPE) is composed of a hexagonal monolayer of epithelial cells. Despite its structural simplicity, the RPE plays an essential role in many cellular processes necessary for visual function.1,2 Both the RPE’s location within the retina and its cellular characteristics directly correlate with its functions, and a breakdown of these processes is implicated in many retinal diseases.1
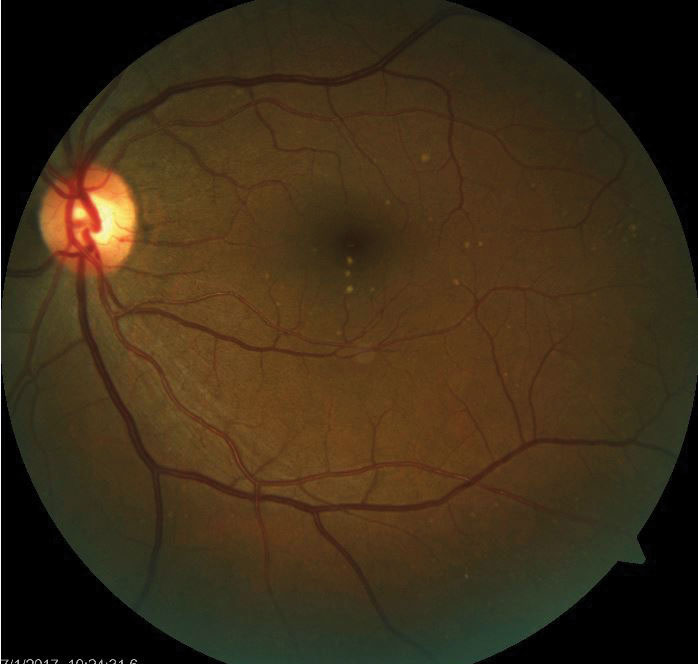 |
| Drusen deposits in the RPE are a harbinger of retinal disease such as AMD. |
Anatomical Overview
The RPE is located between the photoreceptor (PR) layer of the outer retina and Bruch’s membrane.1,2 In the macula, the cell size and shape of the RPE appear flatter and shorter than in the periphery. The apical surfaces of RPE cells contain microvilli that aid in transepithelial transport and the absorption of nutrients from the surrounding extracellular milieu.
The lateral surfaces of the cells are bound together through tight junctions, which are integral, considering the RPE is part of the blood-retinal barrier.1-3 RPE cells are also coupled through gap junctions in other areas, allowing for the diffusion of gases and nutrients across the choroid to the PR. This facilitates the oxygen supply for PR function and aids in the transport and storage of retinoids necessary to maintain the visual cycle.
Composition
The “pigment” of the RPE is due to high amounts of melanin granules with greatest density in the posterior pole and macular region, giving rise to the darker coloration on fundus examination.1 The melanin’s primary function is to provide a barrier against reflected light that would otherwise degrade vision.3 There are also phagosomes, lysosomal enzymes and receptor molecules tasked with the signaling of phagocytosis of primarily rod outer segments, which is necessary for the visual cycle. This metabolic activity is higher in the macula, as is the ratio of PR cells per RPE.1
The RPE is in frequent exposure to visible light for absorption and is more susceptible to reactive oxygen species formation and oxidative damage.1,2 To counterbalance this, the RPE contains antioxidants—a protective measure that declines with age and disease processes.1 Finally, the immunosuppressive factors housed in the RPE contribute to the eye’s immune privilege.2
When Disease Strikes
Disruptions in RPE structure and function result in many retinal diseases. A disturbance of RPE melanin during development results in ocular or oculo-cutaneous albinism. In the aging eye, material may deposit between the RPE and Bruch’s membrane, known as drusen.3
More importantly, the RPE is implicated in age-related macular degeneration (AMD).4 In the early, non-exudative form, oxidative stress and inflammation leads to diminished RPE function and degradation, causing drusen and RPE cell death.5,6
The RPE is also involved in diabetic retinopathy, as prolonged hyperglycemia impedes nutrient and water transport across the RPE. Hyperglycemia is also associated with the reduction of antioxidant activity in the RPE, leading to higher levels of cell damage.2
Understanding how this simple layer works diligently to maintain the processes necessary for visual function is essential because any disruption through disease and aging can compromise retinal integrity.
1. Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and aging changes. Eye. 2001;15:384-89. 2. Simo R, Villarroel M, Corraliza L, et al. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier – implications for the pathogenesis of diabetic retinopathy. J Biomedicine and Biotechnology. 2009;2010:1-15. 3. Sparrow JR, Hicks D, Hamel CP. The retinal pigment epithelium in health and disease. Curr Mol Med. 2010;10(9):802-23. 4. Kauppinen A, Paterno JJ, Blasiak J, et al. Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci. 2016;73:1765-86. 5. Kaarniranta K, Tokarz P, Koskela A, et al. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol Toxicol. 2017;33:113-28. 6. Curcio C, Zanzottera EC, Ach T, et al. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;57(6):BIO211-BIO226. |

