 |
Myasthenia gravis (MG) is an autoimmune disease that affects the neuromuscular junction and is characterized by fluctuating weakness of the skeletal (voluntary) muscle groups of the body.1,2 Its name comes from the Greek and Latin words meaning grave muscular weakness.1 Myasthenia gravis is now recognized as one of the best-defined forms of autoimmune disease.
Ocular myasthenia gravis is a form of the disease in which the extraocular muscles and those muscles that control the eyelids are easily fatigued and weakened.2 Ptosis and extraocular muscle motility deficits are often the first indicators of MG.1 Ninety percent of patients with generalized myasthenia also have ocular involvement. However, in 20% to 30% of MG patients, the condition is confined to the eyes.
Other muscles, such as those that control facial expression, chewing, talking and swallowing, are often involved in the disorder. The prevalence of MG in the United States is estimated to be about 20/100,000, although it is likely under-diagnosed.1-3 MG can occur in all races, both genders and at any age. It most commonly affects young adult women under 40 and older men over 60.1-3
Case Summary
A healthy 59-year-old white male presented with complaints of right upper eyelid droop and painless diplopia on right lateral and up-gaze of five month’s duration. When diplopic, he reported that the two images were displaced vertically. His symptoms tended to be worse in the evening. Upon further probing, the patient reported some degree of weakness and fatigue in his arms and legs.
The Neuromuscular Junction
|
Ophthalmic workup revealed bilateral superior lid ptosis (worse OD) that increased after sustained up-gaze. Binocularity testing revealed a right hypotropia and underaction of the right eye in superior right gaze. Application of an ice pack to the right eye for five minutes led to subjective resolution of the diplopia and an objective improvement in both the ptosis and extraocular movements.
A tentative diagnosis of ocular MG with suspected general MG was established. The patient was referred to a neurology clinic, and acetylcholine receptor (AchR) autoantibodies were subsequently found to be positive. He was successfully treated with pyridostigmine, which relieved the signs and symptoms.
Causes and Symptoms
In most cases of MG, the etiology occurs from autoantibodies binding to acetylcholine receptors at the neuromuscular junction. Cellular immune responses against the receptor are also found.2,3 Complement fixation may occur, causing symptoms by direct injury to the post-synaptic membrane, degradation of the receptors themselves or inhibition of the binding and function of acetylcholine.2
The importance of T cells in the pathogenesis of MG is becoming increasingly apparent. The thymus is the central organ in T cell–mediated immunity, and thymic abnormalities such as thymic hyperplasia or thymoma are well recognized in myasthenic patients.
 | |
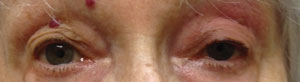 | |
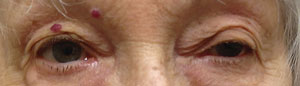
| Above, myasthenia gravis OS. Below, ptosis improves a little after the ice pack test. Photos: Michael Trottini, OD. |
Put the Freeze on Ocular MG
Establishing a diagnosis of isolated ocular myasthenia can be difficult. Symptomatic response to acetylcholinesterase medications may be equivocal, and AChR auto-antibodies may be absent in up to 30% of those who exhibit ocular symptoms for more than two years. No test for myasthenia gravis is 100% sensitive or specific.3
In 1960, Scottish neurologist John A. Simpson hypothesized that MG “is an ‘auto-immune’ response of muscle in which an antibody to end-plate protein may be formed.”4 The clinical observation that symptoms may improve with cold and worsen with heat and the electrophysiological finding that neuromuscular transmission may improve with local cooling form the rationale for the use of the ‘ice pack test’ in the diagnosis of MG.5 The ice pack test is performed by applying an ice pack (or holding an ice cube wrapped in a towel) over the levator palpebrae superioris muscle of a ptotic eye for two to five minutes.
A number of studies have reported improvement of myasthenic ptosis following an ice pack test.6,7 The test is both sensitive and specific for the diagnosis of myasthenia, having no effect on ptosis of other causes, such as oculomotor nerve palsy.
The exact mechanism by which cooling improves myasthenic muscle function has not been completely explained. Cooling is believed to affect the neuromuscular junction both by decreasing cholinesterase activity and by prompting efficacy of acetylcholine at eliciting depolarizations at the end plate.1,2,6,7 Researchers in 1979 reported that myasthenic ptosis improved transiently in six eyes after application of ice for five to 10 minutes. Two patients with non-myasthenic ptosis did not show signs of improvement.8
Common Symptoms of MG
|
More recent research tested 156 patients with an ice pack and edrophonium (Tensilon) with an interval of 15 minutes. Patients were instructed to hold an ice-filled plastic glove on the closed ptotic eyelid. Before and after two minutes of ice application, the distance between the upper and lower margin was measured. Patients with positive test results—an increase of 2mm or higher—were considered controls. The ice pack test was positive in all 61 patients who had a positive Tensilon test and in none of the 95 patients with a negative Tensilon test.9
If you suspect myasthenia gravis, as you should for patients with ptosis, diplopia or both, the ice pack test is a simple, fast, specific and sensitive option for diagnosis.
In Part 2, we will discuss the diagnosis and treatment of MG.
1. Miller NR, Newman NJ. The essential clinical neuroophthalmology. 5th ed. Philadelphia: Lippincott; 1999.2. Benatar M. A systematic review of diagnostic studies in myasthenia gravis. Neuromuscul Disord. 2006 Jul;16(7):459-67.
3. Vincent A, Newsom-Davis J. Acetylcholine receptor antibody as a diagnostic test for myasthenia gravis: results of 153 validated cases and 2,967 diagnostic assays J Neurosurg Psychiatry. 1985;48:1246-52.
4. Simpson JA. Myasthenia gravis: a new hypothesis. Scott Med J. 1960;5:419-36.
5. Borenstein S, Desmedt JE. Temperature and weather correlates of myasthenic fatigue. Lancet. 1974 Jul;2(7872):63-6.
6. Sethi KD, Rivner MH, Swift TR. Ice pack test for myasthenia gravis. Neurology. 1987;37:1383-5.
7. Ertas M, Arac N, Kumral K, Tuncbay T. Ice test as a simple diagnostic aid for myasthenia gravis. Acta Neurol Scand. 1994;89:227-9.
8. Saavedra JS, Femminini R, Kochen S, Ortiz de Zarate JC. A cold test for myasthenia gravis. Neurology. 1979;29:1075.
9. Tabassi A, Dehghani A, Saberi B. The ice test for diagnosing myasthenia gravis. Acta Medica Iranica. 2005;43(1):60-2.
.jpg)

