Despite effective prevention and treatment, the twin epidemics of diabetes and diabetic retinopathy (DR) continue to flourish throughout most of the world.
The Centers for Disease Control (CDC) now estimates 40% of all American adults will be affected by diabetes.1 Of adults with the disease, 28.5% are now diagnosed with DR or diabetic macular edema (DME), or both.2 That’s more than eight million Americans.
Luckily, doctors today are better prepared to face these challenges, with a collection of preventative strategies, diagnostic devices and treatment modalities our predecessors could only imagine. This article reviews the latest thinking and technological advancements in preventing, diagnosing and treating these connected conditions.
Understand Your Goals
Optometrists are the primary eye care providers for a majority of Americans with, and at risk for, diabetes. Of course, not all diabetic retinopathy is created equal; our goal as physicians is to prevent patients with diabetic eye disease from progressing to the level of sight-threatening retinopathy (STR).
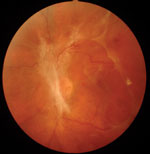
 | |
| Primarily peripheral lesions in a patient with moderately severe nonproliferative diabetic retinopathy. This patient has high risk of progression and proliferative disease, based on recent analysis. Photo: Optos. |
Disease duration and metabolic control of diabetes are the two primary factors affecting development and progression of DR. The longer we delay (or, ideally, prevent) disease onset, and the more we help patients maintain optimal blood glucose, pressure and lipids, the more likely we are to reduce the burden of DR on individuals, communities and our economy.
Focus on Prevention
Obviously, patients won’t develop STR, or go blind from diabetes-related eye disease, if they never develop diabetes in the first place. Surveys show that patients fear vision loss as much as or more than any other disability.3 Optometrists are often an entry point for patients who otherwise may not receive routine health care and can influence patient attitudes and behaviors by explicitly linking prevention of vision loss to prevention of diabetes through healthy lifestyle choices. The Diabetes Prevention Program (DPP) showed that 150 minutes of walking per week significantly reduces the risk of developing type 2 diabetes in high-risk patients by 58% over four years, and by 38% at 10 years.4 Given that diabetes care cost the US economy $245 billion in 2012, pushing diabetes onset 10 years into the future is estimated to save our economy $8 trillion (in 2012 dollars) over the next decade.5
Recommending use of a pedometer with a daily goal of 5,000 to 10,000 steps is a great way to help prevent diabetes, and has been shown to lower hemoglobin A1c about one point.6
Environmental Factors
In addition to the positive influences that healthy lifestyle choices confer, there are of course other environmental factors with negative associations linked to diabetes risk. Notable culprits include added dietary sugars (1.1% increased population prevalence of diabetes—equivalent to 3.5 million additional cases of type 2 diabetes in the United States for every per capita can of sugar-sweetened beverage consumed, according to the International Diabetes Federation), short sleep cycle (threefold increased risk for developing impaired glucose tolerance, a fundamental marker of insulin resistance, when sleep duration is less than six hours and twice the risk with sleep duration above nine hours) and vitamin D insufficiency (studies show an 80% risk reduction when vitamin D blood levels are 53ng/ml vs. 22ng/ml).7-9
New Diabetes Medications • Glucagon-like peptide-1 (GLP-1) analogs, Byetta and Bydureon (exenatide, AstraZeneca) Victoza (liraglutide, Novo Nordisk) and Trulicity (dulaglutide, Eli Lilly) are injected, non-insulin medications that not only lower A1c but also promote weight loss and may reduce cardiovascular risk. • Dipeptidyl peptidase-4 (DPP-4) inhibitors are oral medications that block the enzyme that degrades endogenous GLP-1, but don’t cause weight loss. Januvia (sitagliptin, Merck), Onglyza (saxagliptin, AstraZeneca) and Tradjenta (linagliptin, Boehringer Ingelheim Pharmaceuticals) are examples of DPP-4 inhibitors. • Sodium glucose transporter-2 (SGLT2) inhibitors are oral agents that prevent re-absorption of serum glucose in the kidneys, thereby promoting urinary excretion as well as weight loss and reduction in blood pressure. Examples of SGLT2 inhibitors include Invokana (canagliflozin, Janssen Pharmaceuticals) and Farxiga (dapagliflozin, AstraZeneca). In addition to lowering blood glucose, the weight-loss benefit of these drugs may assist in prevention of STR, as proliferative diabetic retinopathy (PDR) rates are significantly increased when body mass index is above 30kg/m2 (conversely, insulin and sulfonylureas increase weight).18,19 |
In addition to significantly increasing risk of severe age-related macular degeneration, cigarette smoking has also been linked to a 53% increased risk of type 2 diabetes by the US Surgeon General, independent of age, body weight, hypertension and family history.10 Consistently across all studies, greater adherence to a healthy (predominantly plant-based) diet, regular physical activity, avoidance of smoking and moderate consumption of alcohol reduce the risk of developing type 2 diabetes (16% risk reduction for each lifestyle factor).11
The Vigilant Optometrist
Eight million Americans have undiagnosed diabetes and another 86 million are at significant risk. Optometrists can assist in the early identification of diabetes by being vigilant for ophthalmic symptoms and signs like refractive fluctuation, ocular surface disease, recurrent staphylococcal lid disease, dermatologic changes like acanthosis nigricans and, of course, unexplained retinopathy. Other strategies include use of simple, validated in-office screening tools for undiagnosed diabetes like that available from Weill-Cornell Medical College and use of new technology to measure advanced glycation endproducts (AGEs) in the crystalline lens, a biomarker for long-term glucose toxicity that portends diabetes onset as well as complications.12,13
Of note, any random blood glucose value >100mg/dl, an oft-ignored result in patients’ medical records, was recently shown to increase the risk of undiagnosed diabetes 20-fold.14 In-office measurement of spot blood glucose or A1c is another way ODs can help with earlier detection in high-risk patients, depending on each state’s scope-of-practice rulings.
Preventing STR
We can also help our patients who are already diagnosed with diabetes to avoid vision loss by reducing the risk of DR incidence and progression.
Improved blood sugar control has a major impact on diabetic retinopathy, as does improved control of hypertension and, to a lesser extent, dyslipidemia.
Optometrists should extol the “ABCs” of good diabetes management:
• A1c
• Blood pressure
• Cholesterol
• Smoking avoidance (and assessment for sleep apnea)
We also need to recognize the new mantra of diabetes care: individualization of glucose targets. While an A1c value of less than 6.5% might be appropriate for younger adults with no heart disease and long life expectancy, it can actually put older patients with long diabetes duration and cardiovascular disease at substantial risk of death.16
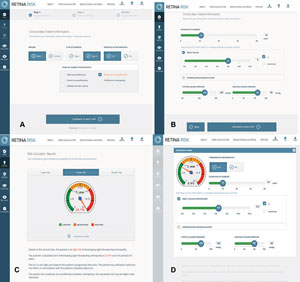 | |
| (A, B) Male patient with type 2 diabetes for 10 years, moderate NPDR, A1c=8% and BP=140/90. (C) Five-year risk for STR is high at 24.1%. (D) This patient’s five-year risk for STR drops dramatically to 6.4% when A1c drops to 6.5% and BP drops to 120/80 using the interactive mode. |
The best time to optimize blood sugar levels is as soon after diagnosis as possible, as this promotes protective metabolic memory (the so-called “legacy effect”) over time that lowers the risk of retinopathy progression, development of STR and other potential complications from diabetes.16,17
Monitoring
We can help patients on insulin therapy achieve better blood glucose control by discussing the advantages of continuous glucose monitoring systems (CGMS). These measure and plot interstitial glucose every five minutes and have audible and vibratory threshold alarms when blood glucose levels are too high or too low. Studies show that CGMS results in improved A1c values for patients with type 1 diabetes, and these may also help prevent fatalities in hypoglycemia-unaware patients such as children, longstanding diabetes patients, the elderly and patients living alone.20
Newer CGMS deliver data to patients’ insulin pumps and suspend delivery of insulin if blood glucose drops below a predetermined threshold, and a dual chamber insulin and glucagon pump is in clinical trials. Also, Google has partnered with Novartis to develop a continuous tear-glucose sensing contact lens.
As the presence of any DR increases the odds of developing sight-threatening retinopathy, early diagnosis of nonproliferative diabetic retinopathy (NPDR) affords us the chance to collaborate with both patients and their other doctors to mitigate that risk. In addition to improved metabolic control, research shows that use of angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blocker (ARB) drugs (-prils and -sartans) lowers progression of DR in hypertensive patients and that the triglyceride lowering drug fenofibrate (found in the brands Tricor, Trilipix and Lipidil) not only lowers risk of DR progression but also the need for laser or anti-vascular endothelial growth factor (anti-VEGF) treatments in type 2 diabetes patients with pre-existing NPDR.21,22 In fact, fenofibrate has now been approved as first-line therapy for NPDR in Australia for this group of patients.23
Nutritional Supplements
A number of studies show that diabetes affects visual function (contrast and visual field sensitivity, color vision, multifocal ERG) long before the appearance of DR, and that macular pigment optical density (MPOD) is reduced in diabetes and even more so in DR.24-29
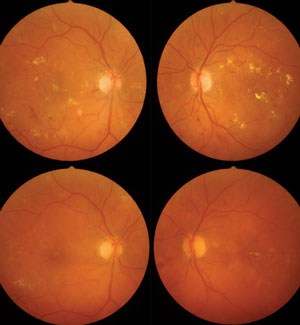 | |
| A 52-year-old patient with type 2 diabetes diagnosed five years ago presenting with center-involved DME, moderate NPDR and 20/200 BCVA in both eyes had received focal laser 16 months prior, but failed to follow-up with retinal specialist claiming Tx “didn’t help” (top panels). Five injections of ranibizumab over 10 months resulted in 200µm reduction in central macular thickness, significant reduction in hard exudate and retinal hemorrhage and 20/60 BCVA in both eyes (bottom panels). At initial exam, A1c was 10.2%. The patient was referred to endocrinology with A1c reduction to 7.5% on insulin therapy. A sleep study confirmed obstructive sleep apnea. | |
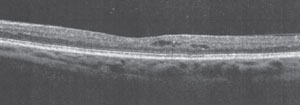 | |
| A 52-year-old patient diagnosed with type 2 diabetes eight years ago, with 20/20 BCVA in both eyes and SD-OCT showing subclinical DME with perifoveal cysts. |
Considerable work with animal models shows specific micronutrients (such as vitamins B, C, D, E, lipoic acid, lutein and zeaxanthin, resveratrol, curcuminoids, benfotiamine and pycnogenol) improve visual function and reduce cellular damage. Recently, a six-month randomized clinical trial (called the Diabetes Visual Function Supplement Study, or DiVFuSS) was conducted to assess possible benefits of a multicomponent nutritional supplement in human subjects with diabetes and early NPDR.30 Results of DiVFuSS show that the test formula significantly improved contrast sensitivity, visual field, color vision, MPOD, symptoms of diabetic peripheral neuropathy and high-sensitivity C-reactive protein (hsCRP) compared with placebo, without affecting A1c levels.30
Improved Detection
Evidence shows that even retina specialists may fail to detect cases of early NPDR, using ETDRS seven-field stereoscopic fundus photography as the gold standard.31 This makes a strong case for use of digital retinal photography and red-free viewing to increase detection of subtle changes. Moreover, an estimated 30% of patients with NPDR have retinal abnormalities primarily outside the posterior pole, a finding that underscores the importance of examining the mid-peripheral and peripheral retina for retinopathy in every patient with known or suspected diabetes, and the additional value of ultrawidefield imaging.32,33 Of high significance, a recent analysis found that patients with NPDR lesions (intra-retinal hemorrhage/microaneurysm, vein beading, IRMA) predominantly in the periphery (outside the standard ETDRS fields) were 3.2 times more likely to have a two-step worsening and 4.7 times more likely to progress to proliferative retinopathy in the course of four years.34
We concentrate on the posterior pole because of the perception that “that’s where the action is”—but we need to focus outside the poles as well.
Buying In
Of course, we can give diabetes patients the best eye examination and treatment plan in the galaxy, but the real question is: How do we get patients to “buy in” to our recommendations for improved self-care?
A clinically validated, visually evocative risk calculator for progression to STR is now available.35 The calculator allows us to demonstrate to patients how better control of their diabetes, both blood glucose and blood pressure, reduces the risk of severe retinopathy. Entering a few key variables that modulate roughly 80% of total risk (diabetes duration and type, presence of any DR, mean blood glucose, blood pressure, gender) provides individualized risk over one, five and 10 years. Using the interactive mode allows us to demonstrate immediately how improved metabolic control lowers each patient’s risk.
Several colleagues and I have found this tool extremely effective for patient understanding and motivation. Combined with retinal imaging and a motivational interviewing strategy, it is now rare for patients to not “buy in” to any of my recommendations.
New Concepts in Diagnosis and Treatment
Careful clinical exam and, sometimes, grading of ETDRS stereo photographs are still the mainstays for diagnosing PDR. Florid neovascularization, fibrovascular proliferation and preretinal or vitreous hemorrhage are generally easy to detect, but detecting subtle disc, retinal or iris neovascularization can be quite challenging. That conundrum has prompted interest in probability-based, automated detection software paired with enhanced digital imaging or OCT.39,40 Hyperreflective neovascular complexes on spectral domain optical coherence tomography (SD-OCT) have been described in subclinical neovascularization of the disc and neovascularization elsewhere, and may soon assist us with identifying proliferative disease in its earliest stages, when treatment is most effective.41 Researchers are looking into using micro-RNA analysis to identify early proliferative disease. The expectation is that serum proteins can be used as biomarkers to detect proteins specific to pathologic retinal neovascularization. Emerging evidence suggests hyperoxygenation of retinal circulation, particularly retinal venules, also is a harbinger of PDR.42,43 Though SD-OCT is the most sensitive instrument for detection of DME, results do not always indicate which patients do and don’t require therapy. However, patients identified with subclinical DME by SD-OCT are three times more likely to develop clinically significant macular edema over two years.44 About one in five patients with DME evaluated by OCT are thought to have vitreomacular adhesion (VMA) that may aggravate the condition.45 These patients may achieve poor visual outcomes with anti-VEGF injections and may benefit from vitrectomy or, anecdotally and off-label, the injected, vitreolytic enzyme Jetrea (ocriplasmin, ThromboGenics).46,47 The evidence suggests our diabetes patients will benefit from routine OCT examination. |
STR Detection and Referral
To detect sight-threatening retinopathy, doctors must start with a thorough clinical examination through dilated pupils. Our index of suspicion should be elevated when patients have a higher risk profile (high A1c, long diabetes duration, type 1 diabetes, uncontrolled hypertension, untreated sleep apnea, clinical depression, and presence of other diabetes complications).36 Though reports suggest the risk of DME increases for those using insulin-sensitizing thiazolidinediones (Actos and Avandia), it appears to be a dose-related phenomenon and is magnified in patients on concomitant insulin therapy.37 Of note, use of ACE inhibitors also significantly attenuates this specific risk.
Therapies
Panretinal photocoagulation remains the therapy of choice for PDR, though anti-VEGF therapies and intravitreal steroids show promise as adjunct treatments.48
Both Lucentis (ranibizumab, Genentech) and Eylea (aflibercept, Regeneron) recently gained FDA approval for prevention of NPDR progression in patients with DME.
Anti-VEGF agents have revolutionized care of patients with center-involved DME and clinically significant macular edema (CSME as defined by ETDRS). These agents are now standard of care.49 Though grid and focal laser photocoagulation reduces the risk of further vision loss from CSME, they rarely improve vision. By contrast, anti-VEGF drugs, more often than not, significantly improve vision and reduce intraretinal edema characteristic of DME. Patients almost always require a series of injections and solid evidence shows that earlier treatment results in larger gains in vision.50
Two anti-VEGF drugs are FDA-approved for DME, Lucentis and Eylea, but off-label use of Avastin (bevacizumab, Genentech) is commonplace due to its significantly lower cost. A recently published one-year, head-to-head comparison of these agents for center-involved DME shows that all three improve visual acuity between 10 to 13 ETDRS letters and with equivalent safety.51 Interestingly, Eylea improved vision about five to seven letters more than both Lucentis and Avastin when entering visual acuity was 20/50 or worse, and subjects randomized to the former required one fewer injection and were 19% to 33% less likely to require rescue laser per pre-specified criteria. Note these are one-year data points only.
Five-year follow up of patients receiving Lucentis plus prompt laser vs. deferred laser therapy, shows slightly better acuity with the latter protocol (mean benefit of +2.6 letters, p=0.09) but patients required more injections (17 vs. 13 over five years, with few treatments required after year three).52
DME has a definite inflammatory component, so intravitreal steroids have long been employed, despite cataract formation in phakic patients and substantial risk of glaucoma. Two sustained-release intravitreal steroid implants are FDA-approved for DME—Ozurdex (dexamethasone, Allergan) and Iluvien (fluocinolone, Alimera)—and evidence of cost and efficacy benefit exists with combination therapy (steroid, anti-VEGF, laser or some combination thereof), especially in recalcitrant cases.53
Finally, mounting evidence shows that obstructive sleep apnea syndrome (OSAS) is causally linked to both DR and DME, that patients with OSAS respond less favorably to anti-VEGF treatments, and that treatment with CPAP improves visual acuity in patients with CSME.54,55
Playing Our Part
We can reduce the burdens of diabetes, diabetic eye disease, vision loss and other complications by focusing on prevention of diabetes, prevention of sight-threatening retinopathy, early detection of DR and especially STR with timely referral for appropriate treatment.
Our understanding and discussion of these ‘game-changing’ advancements with both patients and other physicians will help us secure our vital position on the diabetes care team.
Dr. Chous is in private practice with an emphasis on diabetes eye care and education in Tacoma, Wash. He has been a consultant to Bausch + Lomb, Children with Diabetes, dLife–Your Diabetes Life, Freedom Meditech, Kestrel DiabetesSource, Kowa, Optos, Regeneron, Risk Medical Solutions, Vision Service Plan and ZeaVision. Dr. Chous currently serves as the AOA representative to the National Diabetes Education Program, NIH.
1. Lipscombe LL.The US diabetes epidemic: tip of the iceberg.Lancet Diabetes Endocrinol. 2014 Nov;2(11):854-5.2. Centers for Disease Control and Prevention. National diabetes statistics report, 2014. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2014. http://www.cdc.gov/diabetes/pubs/ statsreport14/national-diabetes-report-web.pdf. Accessed February 28, 2015, 2014.
3. Prevent Blindness America Vision Problems in the US, 2008 Update to Fourth Edition 2. The Economic Impact of Vision Problems Prevent Blindness America 3. 2010
4. Hoskin M, Bray G, Hattaway K, et al. Prevention of Diabetes Through the Lifestyle Intervention: Lessons Learned from the Diabetes Prevention Program and Outcomes Study and its Translation to Practice. Curr Nutr Rep. 2014 Dec 1;3(4):364-378.
5. Anderson J. Achievable cost saving and cost-effective thresholds for diabetes prevention lifestyle interventions in people aged 65 years and older: a single-payer perspective J Acad Nutr Diet. 2012 Nov;112(11):1747-54. doi: 10.1016/j.jand.2012.08.033.
6. Funk M, Taylor E. Pedometer-based walking interventions for free-living adults with type 2 diabetes: a systematic review. Curr Diabetes Rev. 2013 Nov;9(6):462-71.
7. Basu S, Yoffe P, Hills N, Lustig R. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS One. 2013;8(2):e57873.
8. Shan Z, Ma H, Xie M, et al. Sleep Duration and Risk of Type 2 Diabetes: A Meta-analysis of Prospective Studies. Diabetes Care. 2015 Mar;38(3):529-537.
9. Diabetes Prevention with Vitamin D? Grassroots Health, accessed at www.grassrootshealth.net/index.php/diabetes-prevention-with-vitamin-d. February 28, 2015
10. US Dept. of Health and Human Services, The Health Consequences of Smoking – 50 Years of Progress: a report of the Surgeon General 2014. Accessed at http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf. February 28, 2015
11. Ardisson Korat A, Willett W, Hu F. Diet, lifestyle, and genetic risk factors for type 2 diabetes: a review from the Nurses’ Health Study, Nurses’ Health Study 2, and Health Professionals’ Follow-up Study. Curr Nutr Rep. 2014 Dec 1;3(4):345-54
12. Bang H, Edwards A, Bomback A, et al. A patient self-assessment diabetes screening score:development, validation, and comparison to other diabetes risk assessment scores. Ann Intern Med. 2009 Dec 1;151(11):775–83.
13. Cahn F, Burd J, Ignotz K, Mishra S. Measurement of Lens Autofluorescence Can Distinguish Subjects With Diabetes From Those Without. J Diabetes Sci Technol. 2014 Jan 1;8(1):43-9.
14. Bowen M, Xuan L, Lingvay I, Halm E. Random Blood Glucose: A Robust Risk Factor For Type 2 Diabetes. J Clin Endocrinol Metab. 2015 Feb 4:jc20144116. [Epub ahead of print]
15. Ismail-Beigi F, Moghissi E, Tiktin M, et al. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011 Apr 19;154(8):554-9.
16. Ranjit U, Anjana R, Mohan V. Importance of controlling diabetes early--the concept of metabolic memory, legacy effect and the case for early insulinisation. Assoc Physicians India. 2011 Apr;59 Suppl:8-12.
17. Pirola L, Balcerczyk A, Okabe J, El-Osta A. Epigenetic phenomena linked to diabetic complications. Nat Rev Endocrinol. 2010 Dec;6(12):665-75.
18. Kaštelan S, Salopek Rabatic J, Tomic M, et al. Body mass index and retinopathy in type 1 diabetic patients. Int J Endocrinol. 2014;2014:387919.
19. Kaštelan S, Tomic M, Gverovic Antunica A, et al. Body mass index: a risk factor for retinopathy in type 2 diabetic patients. Mediators Inflamm. 2013; 2013:436329.
20. Walker T, Yucha C. Continuous glucose monitors: use of waveform versus glycemic values in the improvements of glucose control, quality of life, and fear of hypoglycemia. J Diabetes Sci Technol. 2014 May;8(3):488-93.
21. Wang B, Wang F, Zhang Y, et al. Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015 Feb 5
22. Simó R, Hernández C. Prevention and treatment of diabetic retinopathy: evidence from large, randomized trials. The emerging role of fenofibrate. Rev Recent Clin Trials. 2012 Feb;7(1):71-80.
23. Pharmacy News (Australia). New indication for Lipidil for diabetic retinopathy, available at www.pharmacynews.com.au/news/latest-news/new-indication-for-lipidil-for-diabetic-retinopath. Accessed March 1, 2015.
24. Sukha A, Rubin A. High, medium, and low contrast visual acuities in diabetic retinal disease. Optometry & Vision Science. 2009;86(9):1086–95.
25. Nittala M, Gella L, Raman R, Sharma T. Measuring retinal sensitivity with the microperimeter in patients with diabetes. Retina. 2012 Jul;32(7):1302-9.
26. Andrade L, Souza G, Lacerda E. Influence of retinopathy on the achromatic and chromatic vision of patients with type 2 diabetes. BMC Ophthalmol. 2014 Aug 31;14:104.
27. Bearse M, Adams A, Han Y. A multifocal electroretinogram model predicting the development of diabetic retinopathy. Prog Retin Eye Res. 2006 Sep;25(5):425-48.
28. Davies N, Morland A. Color Matching in Diabetes: Optical Density of the Crystalline Lens and Macular Pigments. Invest Ophthalmol Vis Sci. 2002;43(1):281–9.
29. Lima V, Rosen R, Maia M, et al. Macular pigment optical density measured by dual-wavelength autofluorescence imaging in diabetic and nondiabetic patients: a comparative study. Sallum J. Invest Ophthalmol Vis Sci. 2010 Nov;51(11):5840-5.
30. Chous A, Richer S, Gerson J, Kowluru R. The Diabetes Visual Function Supplement Study (DiVFuSS): Beneficial effects of a novel, multi-component nutritional supplement evaluated in a double masked, double blind, placebo controlled clinical trial. Br J Ophthalmol, publication pending.
31. Vujosevic S, Benetti E, Massignan F, et al. Screening for diabetic retinopathy: 1 and 3 nonmydriatic 45-degree digital fundus photographs vs 7 standard early treatment diabetic retinopathy study fields. Am J Ophthalmol. 2009 Jul;148(1):111-8. doi: 10.1016/j.ajo.2009.02.031. Epub 2009 May 5.
32. Silva P, Cavallerano J, Sun J, et al. Peripheral lesions identified by mydriatic ultrawide field imaging: distribution and potential impact on diabetic retinopathy severity. Ophthalmology. 2013 Dec;120(12):2587-95.
33. Shi L, Wu H, Dong J, et al. Telemedicine for detecting diabetic retinopathy: a systematic review and meta-analysis. Br J Ophthalmol. 2015 Jan 6. pii: bjophthalmol-2014-305631.
34. Silva P, Cavallerano J, Haddad N, et al. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology. 2015 Feb 19. pii: S0161-6420(15)00046-9.
35. Aspelund T, Thornórisdóttir O, Olafsdottir E, et al. Individual risk assessment and information technology to optimise screening frequency for diabetic retinopathy. Diabetologia. 2011 Oct;54(10):2525-32. doi: 10.1007/s00125-011-2257-7. Epub 2011 Jul 27.
36. Yau J, Rogers S, Kawasaki R, et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care. 2012 March;35(3):556–64.
37. Idris I, Warren G, Donnelly R. Association between thiazolidinedione treatment and risk of macular edema among patients with type 2 diabetes. Arch Intern Med. 2012 Jul 9;172(13):1005-11. doi: 10.1001/archinternmed.2012.1938.
38. Karperien A, Jelinek H, Jorge J, et al. Automated detection of proliferative retinopathy in clinical practice. Clin Ophthalmol. 2008 Mar;2(1):109–22.
39. Welikala R, Dehmeshki J, Hoppe A, et al. Automated detection of proliferative diabetic retinopathy using a modified line operator and dual classification. Comput Methods Programs Biomed. 2014 May;114(3):247-61.
40. Miura M, Hong Y, Yasuno Y, et al. Three-dimensional Vascular Imaging of Proliferative Diabetic Retinopathy by Doppler Optical Coherence Tomography. Am J Ophthalmol. 2015 Mar;159(3):528-38.e3.
41 Muqit M, Stanga P. Fourier-domain optical coherence tomography evaluation of retinal and optic nerve head neovascularisation in proliferative diabetic retinopathy. Br J Ophthalmol. 2014 Jan;98(1):65-72. doi: 10.1136/bjophthalmol-2013-303941. Epub 2013 Oct 24.
42. Qing S, Yuan S, Yun C, et al. Serum miRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell Physiol Biochem. 2014;34(5):1733-40.
43. Jørgensen C, Hardarson S, Bek T. The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision-threatening retinopathy. Acta Ophthalmol. 2014 Feb;92(1):34-9.
44. Pires I, Santos A, Nunes S, et al. Subclinical macular edema as a predictor of progression to clinically significant macular edema in type 2 diabetes. Ophthalmologica. 2013;230(4):201-6.
45. Jackson T, Nicod E, Angelis A, et al. Vitreous attachment in age-related macular degeneration, diabetic macular edema, and retinal vein occlusion: a systematic review and metaanalysis. Retina. 2013 Jun;33(6):1099-108.
46. Yoon D, Rusu I, Barbazetto I. Reduced effect of anti-vascular endothelial growth factor agents on diabetics with vitreomacular interface abnormalities. Int Ophthalmol. 2014 Aug;34(4):817-23.
47. de Smet, Castilla M. Ocriplasmin for diabetic retinopathy. Expert Opin Biol Ther. 2013 Dec;13(12):1741-7.
48. Bressler S, Qin H, Melia M, et al. Exploratory Analysis of Effect of Intravitreal Ranibizumab or Triamcinolone on Worsening of Diabetic Retinopathy in a Randomized Clinical Trial. JAMA Ophthalmol. 2013 August; 131(8):1033–40.
49. Boyer D, Hopkins J, Sorof J, Ehrlich J. Anti-vascular endothelial growth factor therapy for diabetic macular edema. Ther Adv Endocrinol Metab. 2013 Dec;4(6):151-69.
50. Brown D., Nguyen Q, Marcus D, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. RISE and RIDE Research Group. Ophthalmology. 2013;120:2013-22.
51. The Diabetic Retinopathy Clinical Research Network. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N Engl J Med. 2015 Feb 18. [Epub ahead of print].
52. Elman M, Ayala A, Bressler N, et al. Intravitreal Ranibizumab for Diabetic Macular Edema with Prompt versus Deferred Laser Treatment: 5-Year Randomized Trial Results. Ophthalmology. 2015 Feb;122(2):375-81.
53. Ciulla T, Harris A, McIntyre N, Jonescu-Cuypers C. Treatment of diabetic macular edema with sustained-release glucocorticoids: intravitreal triamcinolone acetonide, dexamethasone implant, and fluocinolone acetonide implant. Expert Opin Pharmacother. 2014 May;15(7):953-9.
54. Nesmith B, Ihnen M, Schaal S. Poor responders to bevacizumab pharmacotherapy in age-related macular degeneration and in diabetic macular edema demonstrate increased risk for obstructive sleep apnea. Retina. 2014 Dec;34(12):2423-30.
55. Mason R, Kiire C, Groves D, et al. Visual improvement following continuous positive airway pressure therapy in diabetic subjects with clinically significant macular oedema and obstructive sleep apnoea: proof of principle study. Respiration. 2012;84(4):275-82.