 |
Glaucomatous optic neuropathy is a disease we all encounter in our daily practices, with an estimated 64 million people affected.1-3 As one of the leading causes of vision loss worldwide, early detection is vital in establishing a treatment plan to reduce the risk of progression. Damage from glaucoma can be traced back to one essential retinal neuron: the ganglion cell.1-6
Follow the Light
When light enters the eye, it passes through the cornea, aqueous, pupil and vitreous to land on the neural retina. The group of neurons that constitutes the retina includes photoreceptors and bipolar, horizontal amacrine and ganglion cells. One of the larger neurons is the retinal ganglion cell (RGC), which resides within the inner retinal layers.3
The RGCs are the sole efferent fibers within the retina—their intricate network of dendrites receives input and ultimately transmits the signal to the necessary areas of the brain. Thousands of RGC axons travel towards the center of the retina, converging at the optic nerve head. They then exit the lamina cribrosa and pass their information to the lateral geniculate nuclei within the thalamus for visual stimulation.3
There are two main groups of RGCs: (1) midget cells, which are responsible for the P visual pathway and (2) parasol cells, which are responsible for the M pathway. They differ in their receptive field size, luminance, contrast and spectral sensitivities (Table 1).4
RGCs can be further classified based on their response to light with respect to the receptive fields. ON cells are excited by increments of light while OFF cells are excited by decrements of light. ON-OFF RGCs are a mixture of both.2
Studies determining susceptibility of specific RGCs and glaucoma have found that larger parasol cells are more prone to IOP-induced injury.4 No distinction was found regarding the difference between ON/OFF cell susceptibility to glaucomatous damage.2
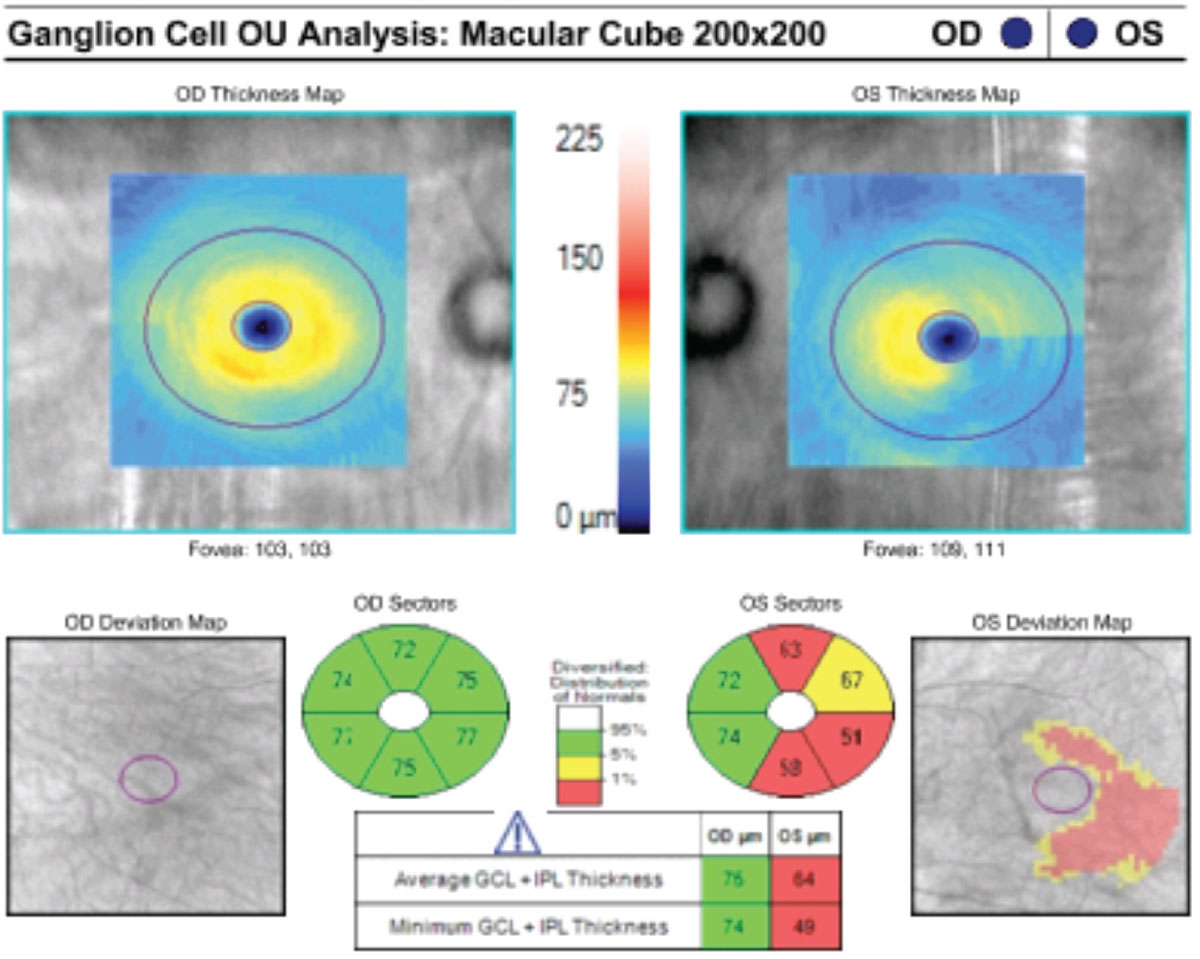 |
This patient’s macular GCC OCT indicates reduced GCL and IPL thickness in their left glaucomatous eye. Click image to enlarge. |
Tracing Cell Death
RGC destruction is the hallmark of glaucoma and other optic neuropathies, such as Leber’s hereditary optic neuropathy, optic neuritis and ischemic optic neuropathy, to name a few.3 With glaucoma, research shows RGCs are the most susceptible neurons to stressors such as intraocular pressure (IOP).4 Increases in IOP lead to a reduction in retrograde axonal flow, causing death.5 Due to the RGC pathway, cell death leads to optic nerve degeneration and subsequent visual field loss.
Table 1. Retinal Ganglion Cells by Type | |
| Midget Cell | Parasol Cell |
| P visual pathway | M visual pathway |
| Small receptive fields | Large receptive fields |
| Decreased luminance sensitivity | Increased luminance and contrast sensitivity |
| Spectral sensitivity | No spectral sensitivity |
Research estimates that 25% to 40% of RGCs are lost before a visual field defect is evident, emphasizing the importance of careful examination of the innermost retinal layers.5,6
Traditional optical coherence tomography (OCT) of the circumpapillary optic nerve has become essential in quantifying structural loss of RGCs within the retinal nerve fiber layer (RNFL), as it is the first detectable sign of glaucoma. However, the RNFL contains only the axonal portion of the RGC.6 The remaining portions of the RGC—the cell body and dendrite—are located within the ganglion cell layer (GCL) and the inner plexiform layer (IPL), respectively.
The Nerve of These CellsWhile glaucoma is the most common disorder, RGCs are affected in many other optic nerve diseases as well. Neuro-ophthalmic disorders, such as dominant optic atrophy, affect the RGCs because they originate primarily from the diencephalon of the central nervous system. Clinically, this optic atrophy appears as gradual, bilateral significant vision loss that manifests early in life and is characterized by optic nerve pallor.1 Leber’s hereditary optic neuropathy (LHON) is another example. It’s a genetic disorder primarily affecting males that causes painless and acute monocular vision loss. LHON pathology is confined to the RNFL, affecting the RGCs. The GCC OCT is useful in diagnosing these patients and monitoring for progression, with typical patterns reflecting early thinning within the inner ring of the nasal sector, progressing to a centrifugal and spiral pattern of RNFL and IPL thinning.2 The GCC is also helpful in distinguishing between optic neuritis and non-arteritic ischemic optic neuropathy (NAION), as both conditions present initially with optic nerve swelling. In the GCC of NAION patients, the ganglion cell loss generally follows an altitudinal pattern, consistent with the subsequent visual field defect; whereas, ganglion cell damage in optic neuritis is more diffuse.3 Even in compressive optic neuropathies, where a mass in the brain is the cause such as in a pituitary adenoma, the lesion may inhibit nerve impulses of RGC axons. Often, following decompression, GCC thinning may remain although vision is restored. This can be attributed to the temporary, physiological block in nerve conduction without the death of RGC axons.4
|
RGC death dynamics suggest that mitochondrial changes that harm the dendrites within the IPL layer occur first, followed by the GCL and RNFL. These findings have given rise to the utility of the OCT macular ganglion cell complex (GCC) map, which analyzes all three layers of the retina and, ultimately, the entire structure of the RGCs. The GCL+IPL measurements incorporate the cell body and dendrite as well; whereas, the standard RNFL map only measures RGC axons.5
The GCC also provides clinicians the capability to measure macular thickness, which may be reduced in glaucoma due to the high distribution of RGCs within the macular complex. Studies demonstrate that this specific type of OCT is quite useful when attempting to distinguish glaucomatous damage in a patient with high myopia. This is because myopic optic discs are difficult to distinguish in RNFL measurements alone due to the presence of peripapillary atrophy, staphylomas or tilted insertions.6
RGCs are vital to the visual pathway, and their destruction is a key mechanism of glaucomatous optic neuropathy. With a better understanding of the complex structure of RGCs and their role in glaucoma, researchers have been able to improve our imaging modalities to help us detect glaucomatous changes earlier, even preceding vision loss.
The early and subtle degenerations to RGCs must be identified to initiate IOP-lowering treatment in glaucoma patients and preserve visual function.
1. Ratican SE, Osborne A, Martin KR. Progress in gene therapy to prevent retinal ganglion cell loss in glaucoma and Leber’s hereditary optic neuropathy. Neural Plast. 2018;2018:7108948. 2. Risner ML, Pasini S, Cooper ML, et al. Axogenic mechanism enhances retinal ganglion cell excitability during early progression in glaucoma. Proc Natl Acad Sci. 2018;115(10):E2393-402. 3. Miltner AM, La Torre A. Retinal ganglion cell replacement: current status and challenges ahead. Dev Dyn. 2019;248(1):118-28. 4. Della Santina L, Ou Y. Who’s lost first? Susceptibility of retinal ganglion cell types in experimental glaucoma. Exp Eye Res. 2017;158:43-50. 5. Dascalescu D, Corbu C, Coviltir V, et al. The ganglion cell complex as an useful tool in glaucoma assessment. Rom J Ophthalmol. 2018;62(4):300-03. 6. Scuderi G, Fragiotta S, Scuderi L, et al. Ganglion cell complex analysis in glaucoma patients: what can it tell us? Eye Brain. 2020;12:33-44. |

