No definitive test for dry eye disease (DED) currently exists, and it is likely that we will never see a “magic bullet” of this sort. In fact, the current prevailing definition of dry eye from the DEWS report in 2007 is very broad, encompassing a multitude of symptoms and signs.1 Dry eye is actually much more complicated than we have historically given it credit for. The term more accurately describes a diverse group of distinct conditions with unique etiologies; as such, several different pathologies lead to the same signs and symptoms in our patients. The search for a single, simple, absolute test for dry eye has given rise to an ever-expanding clinical armament of tests that measure specific hallmarks of dry eye disease.
But why? Likely this is because our rapidly changing understanding of dry eye pathology has driven the development of new DED diagnostic technologies. We have seen the dry eye paradigm shift several times as new theories have supplanted their predecessors. After first relying on the concept of primary aqueous deficiency, we adopted a theory of primary lipid deficiency, before moving to a combined aqueous-lipid deficiency model and finally the current tear composition and comorbidity paradigm. New advances in knowledge have shifted the diagnostic goal post several times. Importantly, however, the tenet that inflammation is a key component of all dry eye has remained intact throughout these regime changes in theory.8
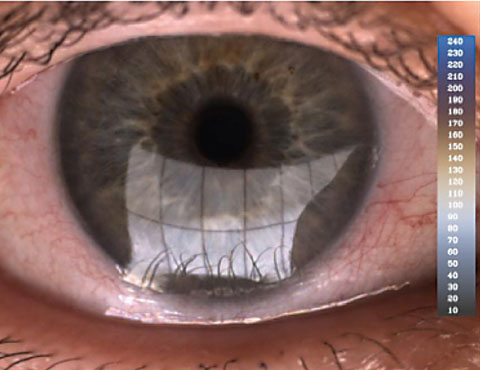 |
| The Lipiview II uses interferometry to assess lipid layer integrity. |
It can be overwhelming to look at the entirety of new technologies, each of which focuses on distinct aspects of DED. Taken individually, the scientific rationale and clinical application of each diagnostic test is relatively straightforward. However, no clear map exists to help differentiate between tests and understand how one test’s interpretation affects another.
Dry eye centers throughout the country have incorporated most—and in many cases all—of the technologies highlighted here, and development of algorithms and protocols for diagnosis are in the works. Here, we will walk you through how to incorporate and interpret the current dry eye diagnostic technologies.
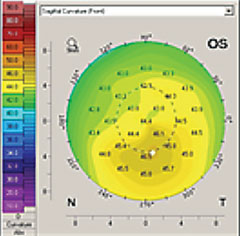 |
| Corneal topography can identify ocular surface changes stemming from dry eye. |
1. Questionnaires
While validated dry eye questionnaires remain staples of clinical research, for the most part they remain underused in general optometric practice. The two most commonly used questionnaires—OSDI and SPEED—are still commonly used in many dry eye centers.1,2 Although we do not use a validated dry eye questionnaire on our routine eye care patients, we do find use in all our dedicated ocular surface disease evaluations. Currently, the SPEED questionnaire is the prime choice; its brevity makes it quick for patients to fill out and for clinicians to evaluate. Whether you choose a validated questionnaire or develop one to fit your practice needs, be sure to use simple, real-world scenarios for patients to think about how dryness impacts their daily activities, how frequently they experience it and how severe their condition may be.
As a part of our routine eye exams, we screen for dry eye with verbal questions rather than questionnaires. The three quick screening questions published by the 2014 Dry Eye Summit have been incorporated into every initial patient encounter we have.3 Although not validated, these questions have proven helpful to us in identifying dry eye quickly and in a minimally disruptive manner during our surgical evaluations.
1. Do your eyes ever feel dry or uncomfortable?
2. Are you bothered by changes in your vision throughout the day?
3. Do you ever use or feel the need to use drops?
2. Corneal Topography
Topography, which can detect subtle irregularities to the ocular surface, is an excellent tool for the detection of dry eye.4 However, note that a diagnosis must be based on medical necessity (e.g., keratoconus or corneal dystrophy) to bill for topography in this context; otherwise, the patient is required to pay out of pocket for the test.
Regardless, the information gleaned can be very useful in DED detection. We frequently perform the test on many of our patients, especially those who are considering cataract surgery, elective procedures like LASIK or PRK, or contact lens wear. Topography can be included as part of the elective procedure or contact lens fitting fees for obvious reasons.
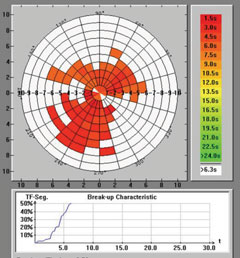 |
| Keratograph 5M evaluation of TBUT. |
Due to the impact of dry eye on refractive outcomes and successful contact lens wear, it is critical to determine the presence or absence of DED in surgical patients. For instance, in refractive cataract surgery, an inaccurate keratometry measurement with biometry can lead to a +/- 1D change in postsurgical refraction. Additionally, contact lens wearers need a robust tear film and healthy ocular surface to ensure successful wear and optimal vision.
A thorough evaluation of the patient’s corneal surface and shape can provide information about the presence and state of DED. Missing or patchy information on topography is often an indicator of early tear break-up time (TBUT). Inferior steepening is also a common result of dry eye, which can become so severe as to mimic keratoconus. The inferior cornea will often steepen in dry eye because of epithelial dehydration.5 One will often see superficial punctate keratitis in the inferior cornea as well. The presence of significant inferior steepening causes us to focus more on exposure-related causes of dry eye such as incomplete blink or nocturnal lagophthalmos.
As dry eye improves, so too will topography. Other topographical findings of dry eye disease include irregularly shaped placido discs and differences in average keratometry readings between eyes.4,5
3. Lipid Layer Analysis
Recent research suggests that 86% of dry eye patients suffer from the evaporative form of the disease.6 Thus, it important that we fully assess the lids, meibomian glands and lipid layer—whose evaluation is now a necessity for determining the presence and severity of dry eye. Fluctuating vision is a hallmark of dry eye and an insufficient lipid layer is believed to be the most likely cause.1 Several automated devices are available on the market to evaluate the lipid layer. If none are available at your clinic, however, fluorescein TBUT is still an excellent alternative. Contrary to conventional wisdom, no ‘magic number’ for TBUT exists. Rather, look to see if the lipid layer is breaking up between routine blinks.
Automated assessments of the lipid layer help us understand its importance. Lipiview II (TearScience) and the Keratograph 5M (Oculus) are two of the most readily available technologies for evaluating the lipid layer. The Lipiview II employs interferometry to evaluate the lipid layer thickness; a thickness of roughly 100nm is considered normal.7 The test is valuable on initial ocular surface disease exams, but we do not yet use it to track the efficacy of dry eye treatments because of interscan variability.
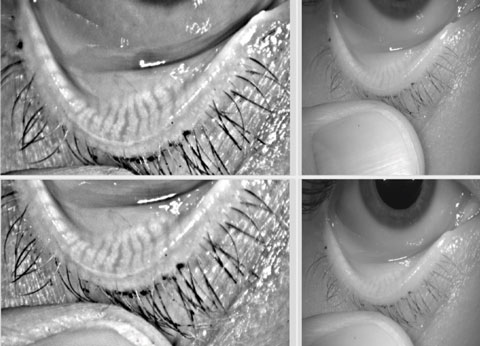 |
| Keratograph 5M image showing healthy meibomian glands. |
The Keratograph 5M evaluates noninvasive TBUT: no fluorescein is needed in the eye, and the natural lipid layer is evaluated for its break- up patterns. This data is useful in tracking patients. The drawback: the numbers are not standardized or validated for diagnosing DED.
A deficient lipid layer will turn treatment focus toward using lipid-containing artificial tears and therapies that increase meibomian gland expression.
4. Automated Blink Analysis
Lipiview II tracks and evaluates patients’ routine blink characteristics, including blink rate and partial or incomplete closure. The test is run with the lipid layer analysis, and we review this data before every dedicated dry eye evaluation. For patients who exhibit incomplete blinks, this is an opportunity to educate them on how this contributes to their condition. They can be shown video of their blink patterns and as such may be more inclined to understand the need for treatment compliance or lifestyle changes. Blinking exercises can be prescribed for incomplete blinkers, but the efficacy of these exercises is debatable.
5. Objective Red Eye Scaling
Chronic red eyes is a common sign of DED. Objectively measuring conjunctival injection allows the symptom to be used as a metric to diagnose, educate and monitor treatment. This can be accomplished with Keratograph 5M, which scores conjunctival redness. The automated software uses a repeatable metric to measure redness, minimizing observer variability. Although causes of red eye are endless, this test may provide a valuable baseline for assessing treatment efficacy.
6. Tear Osmolarity
This may be the single most accurate diagnostic test for dry eye—and the most widely misunderstood.8 Because DED signs and symptoms often do not correlate, having an objective metric generated by the TearLab device improves our ability to rapidly diagnose and treat dry eye. Just as the disease itself may fluctuate (e.g., unstable tears, blurred and fluctuating vision), high osmolarity readings in a dry eye patient will be increased and fluctuate when the tear film is unstable (greater than 308mOsm/L).
Additionally, inter-eye variability is a characteristic of dry eye not seen in normal subjects (greater than 8mOsm/L difference between eyes).9 In combination with the slit lamp exam, doctors can better select therapies based on the mechanism of disease and severity. As we know, tear osmolarity will be altered by any disruption in the tear film; however, the numbers don’t always fit into our conventional thinking of dry eye. Normal patients will exhibit low and stable osmolarity (less than 308mOsm/L), which indicates proper tear homeostasis.9 As the body struggles to maintain homeostasis in dry eye, however, tear osmolarity numbers will become variable and inconsistent. Once we improve the tear film composition, tear osmolarity numbers in DED patients become less variable and more consistent. We use these readings to help us to treat and manage our dry eye patients.
Although tear osmolarity has a high positive predictive value, we do not rely on it as a standalone test—we find it is more valuable to track dry eye therapy over time. As tear osmolarity normalizes (280 to 300mOsm/L), we adjust and monitor our treatment and management accordingly.9 Once we have a patient that is symptomatic or diagnosed with dry eye, we evaluate tear osmolarity at every evaluation thereafter.
 |
| Lipiview II combines reflected and transilluminated light to produce dynamic meibomian imaging. |
Because of the low cost—evaluation units are free of charge—and significant clinical value, we see tear osmolarity as the baseline new technology for evaluating tear film stability. This point-of-care test takes only seconds and can be done by a technician. It is also one of the few dry eye diagnostic tests (e.g., meibography and MMP-9) that is reimbursable by medical insurance.
7. Matrix Metalloproteinase-9 (MMP-9) Analysis
MMP-9 is a proteolytic enzyme released by compromised epithelium, and has been shown to increase in dry eye patients.10 MMP-9 analysis (InflammaDry, RPS) is a point-of-care diagnostic test that provides both qualitative and quantitative information that may aid the diagnosis of DED. Positive readings indicate the presence of inflammation in the eye. A positive result indicates the sample contains more than 40ng/ml, and has been correlated with dry eye of DED.12
MMP-9 is helping us to catch patients we otherwise would have been missing. For instance, we have found presurgical or contact lens patients with a missed DED diagnosis. Additionally, this test is useful for determining the optimal time to use punctal occlusion, since we do not want to put plugs in a patient with an already inflamed ocular surface. The test only takes a few minutes to perform and is reimbursable by most insurers.
8. Schirmer Tear Test
We do not routinely perform this test on all patients, as it is highly variable and does not account for the tear composition or the evaporative nature of dry eye.12 When performing this test, be sure to measure basal tearing—we always perform this test with topical anesthetic. After applying the drop, remove any excess with a cotton swab prior to measurement.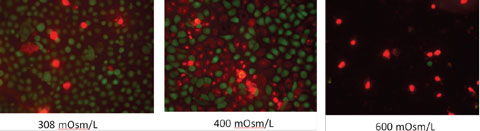 |
| Click image to enlarge. Effect of high osmolarity on corneal epithelial cells (200x magnification). Photos: Tearlab. |
A variation of this test is the phenol red test, which many practitioners find useful. Phenol red is performed without anesthetic and takes about 15 seconds. If the results show less than 10mm of wetting, the patient is considered dry. One area where we find both tests useful is in new dry eye patients and in cases of suspected autoimmune conditions such as Sjögren’s syndrome. If the patient has a low Schirmer score due to aqueous deficiency, we often initiate punctal plugs sooner and order further testing for autoimmune conditions such as Sjögren’s (i.e., Sjö, Bausch + Lomb).
9. Lissamine Green/Rose Bengal Staining
These stains are the most diagnostic for early to moderate dry eye. When evaluating the interpalpebral conjunctiva, positive staining with lissamine green helps to detect the earliest signs of the disease prior to corneal staining. Lissamine green is the vital dye of choice used in symptomatic patients who have not yet started to show corneal fluorescein staining.
Rose bengal staining is analogous to lissamine green, but because it can burn upon instillation, it is used less than its green-colored counterpart.13 Unlike fluorescein and lissamine green, rose bengal staining is not a “vital” dye, as it stains normal, healthy, living cells in addition to dead or dying ones.14,15
10. Sodium Fluorescein (NaFl) Staining
NaFl staining is the most commonly used stain in dry eye evaluation due to its wide availability.12 It is important to remember that NaFl only sufficiently stains the cornea, and only in moderate to severe dry eye. When it comes to diagnosing dry eye, it is critically important to identify DED patients earlier in the disease state. If you are only using NaFl to screen for dry eye, you may be missing most mild to moderate dry eye patients.
The pattern of staining can also play a role in our diagnosis of the condition. Often, patients with dry eye disease have diffuse staining; however, some will only have significant inferior staining. In these cases, always take a look at the lids, lid position and blink to identify other causes of their symptoms.
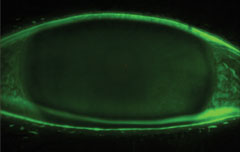 |
| Fluorescein staining of the cornea. |
11. Tear Lake/Meniscus Evaluation
Clinicians interested in obtaining precise measurements of the tear layer thickness or tear lake size can make use of anterior segment OCT. This data can be valuable in certain dry eye evaluations, but is not routinely used to diagnose dry eye due to a lack of standardization.16 It is also typically not reimbursable by insurance. Conventional evaluation at the slit lamp will suffice for most routine cases. However, one area where OCT testing may be useful is in the evaluation of scleral contact lenses for dry eye relief.
12. Meibography
Given our current understanding of how important lipid layer interaction is in almost all presentations of dry eye, meibography has become an essential DED test. As is the case with glaucoma, where we look at structure and function of the optic nerve, our ability to image the meibomian glands has significantly changed how we understand and treat DED.
Although we do not use meibography as a standalone diagnostic test—it does not tell us how much lipid is expressed from each gland—it does give us a general understanding of patients’ gland health, and which patients may benefit from various meibomian gland-based treatments. Meibography also improves our ability to educate patients on the progressive nature of their condition if left untreated. Through years of imaging, we have learned the gland will initially plug, then swell, then become serpiginous; finally, it will truncate and atrophy.17 Knowing the state of patients’ meibomian gland health is invaluable for the long-term management of their dry eye.
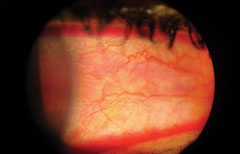 |
| Rose bengal conjunctival staining. |
We perform meibography during all dry eye evaluations, and have started to image the glands of all patients scheduled for LASIK or cataract surgery. Meibography is becoming a valuable screening tool for identifying people at risk for postsurgical dry eye due to poor meibomian gland health. We are also performing meibography on many of our glaucoma patients due to the comorbidity rate associated with glaucoma and DED.
The cost to entry associated with meibography (Keratograph 5M or Lipiview II) is significant, but this imaging technique is becoming increasingly important as we discover more about dry eye. If neither the Keratograph 5M or Lipiview II is available, a rudimentary evaluation of the meibomian glands can be performed by transillumination of the eyelid with a small light source.
Diagnostic Wrap-Up
The expansive technology available to diagnose dry eye yields no single definitive test. Traditional dry eye testing (i.e., history, Schirmer’s, TBUT) is a start; however, the objective tests mentioned here will help not only to identify patients but also to treat and manage DED. Consistency is the key to building your dry eye practice while finding the dry eye protocols that work for your clinic. Asking the right questions, examining the lids, using the stains and assessing tear stability are essential. Dry eye is the most common ocular disease that you will see. Therefore, it should take its appropriate priority in your clinic.
The authors wish to thank Cecelia Koetting, OD, for her assistance with this article.
|
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):75-92. 2. Ngo W, Situ P, Keir N, et al. Psychometric properties and validation of the Standard Patient Evaluation of Eye Dryness questionnaire. Cornea. 2013;32(9):1204-10. 3. Grubbs JR, Tolleson-Rinehart S, Huynh K, Davis RM. A review of quality of life measures in dry eye questionnaires. Cornea. 2014 Feb;33(2):215-8. 4. 2014 Dry Eye Summit. Accessed from www.reviewofoptometry.com/supplement_toc/s/367. 5. Liu Z,Pflugfelder S. Corneal surface regularity and the effect of artificial tears in aqueous tear deficiency. Ophthalmology. 1999;106(5):939-43. 6. dePaiva, CS, Harris, L, Pflugfelder, S. Keratoconus-like Topographic Changes in Keratoconjunctivitis Sicca. Cornea. 2003;22(1):22-4. 7. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-8. 8. Finis D, Pischel N, Schrader S, Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea. 2013;32:1549–53. 9. Tomlinson A, IOVS 2006. DEWS Ocular Surf 2007. Lemp MA, Bron AJ, Baudouin C et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. 2011 May;151(5):792-8. 10. Chotiakavanich S, de Paiva CS, Li de Quan, et al. Invest Ophthalmol Vis Sci 2009; 50(7):3203-9. 11. Sambursky R, Davitt WF 3rd, Friedberg M, Tauber S. Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease. Cornea. 2014;33:812-28. 12. Nichols KK, Mitchell GL, Zadnik K. The Repeatability of Clinical Measurements of Dry Eye. Cornea. 2004;23(3): 272-85. 13. Machado LM, Castro RS, Fontes, BM. Staining Patterns in Dry Eye Syndrome: Rose Bengal Versus Lissamine Green. Cornea. 2009;28(7):732-4. 14. Abelson M, Ingerman, A. The dye-namics of dry-dye diagnosis. Review of Ophthalmology. Access from www.reviewofophthalmology.com/content/d/therapeutic_topics/i/1311/c/25254/#sthash.AxbyGJDn.dpuf. 16. Feenstra RPG, Tseng SCG. Comparison of fluorescein and Rose Bengal staining. Ophthalmology 1992;99:4:605-17. 17. Shizuka Koh, S. Tung, C. Aquavella, J. et al. Simultaneous measurement of tear film dynamics using wavefront sensor and optical coherence tomography. Invest Ophthalmology Visual Science. 2010 Jul; 51(7):3441-8. 18. Nichols JJ, Bernsten DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea. 2005; 24(4):382-8. 19. Patterson M, Vogel H, Prenner EJ. Biophysical characterization of monofilm model systems composed of selected tear film phospholipids. Biochim Biophys Acta. 2016 Feb;1858(2):403-14. doi: 10.1016/j.bbamem.2015.11.025. Epub 2015 Nov 30. |

