As the anterior-most structure of the eye, the cornea plays an important role in vision, the mechanical integrity of the eye and immunological defense. It is highly structured—a trait critical to its abilities to refract light and prevent infection. However, the cornea’s position leaves it susceptible to a variety of injuries and insults. Proper function of the tear film, lids and conjunctiva are all essential in maintenance of the cornea, which leaves corneal function vulnerable to disruption from all corners of the globe—and beyond. Corneal healing mechanisms are in place to aid in proper repair and preservation of corneal structure after injury. And, when these fail, therapeutic advances are available that aim to minimize long term complications.
Corneal A&P
A typical episode of corneal wound healing consists of a complex sequence of events that involves numerous cell types within the appropriate biochemical environment. Maintaining an understanding of the anatomical and physiological characteristics of the cornea—from its multilayer structure down to its cellular junctions—can help practitioners better understand the clinical key points in this process.
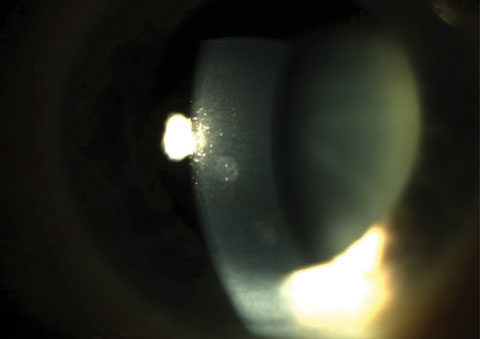 |
| Fig. 1. Two anterior stromal scars. Click image to enlarge. |
From anterior to posterior, five familiar layers comprise the cornea: epithelium, Bowman’s layer, stroma, Descemet’s membrane and the endothelium. A sixth corneal layer, the so-called Dua’s layer or pre-Descemet’s layer, has been proposed as a necessary addition to the list, but its disputed nature means it remains a separate classification for now.1
• Corneal epithelium. Composed of five to seven layers of cells, and approximately 50µm thick, the epithelium contains several cell types oriented in layers from anterior to posterior, paralleling the larger corneal structure itself. Specific cell junctions are responsible for maintaining the relatively dehydrated state of the cornea, for cellular communication and exchange of materials, and for the cornea’s selective permeability. The epithelial cells aid in maintaining a stable tear film, and for secreting the epithelial basement membrane—critical in corneal healing. Corneal epithelial cells are constantly turned over as the outermost cells are shed into the tear film. The entire epithelium is turned over in approximately seven to 10 days. This process is accelerated during wound healing and generally leads to rapid healing for corneal injuries that only involve the epithelial cells.8 (See, “A Closer Look: The Corneal Epithelium”).
A Closer Look: The Corneal EpitheliumThe superficial layer of cells comprising the epithelium is approximately two cell layers thick and composed of non-keratinized squamous cells.2 These cells are fairly flat with numerous microvilli and microplicae, which increase their total surface area to help stabilize the overlying tear film. The epithelial glycocalyx helps adhere the tears to the corneal surface cells, thereby preventing the binding of pathogens.3 These surface cells are joined together by desmosomes as well as tight junctions, which are primarily composed of zonula occludens. The tight junctions help provide a highly selective barrier to substances in the tear film.4 Intercellular junctions help prevent unwanted substances from entering the cornea and aids in maintaining the deturgesence of the cornea. The second layer of the epithelium is composed of two to three layers of wing cells joined together by desmosomes and adherens junctions. They are attached anteriorly to the surface cells and posteriorly to basal cells by desmosomes and also joined together by gap junctions, allowing molecules to be exchanged directly between individual cells The basal cell layer consists of a single layer of columnar cells that form the posterior-most layer of the corneal epithelium. These cells are attached to each other via gap junctions—which play a role in mediating and differentiating intercellular communication—and desmosomes. These basal cells secrete a basement membrane, which is divided into an anterior lamina lucida and a more posterior lamina densa.5 The corneal epithelial basement membrane is composed primarily of collagens, laminins, proteoglycans and nidogens.6 The basement membrane plays an important role in cellular functions, including those involved in healing, by controlling the binding of growth factors and their local concentrations between cell layers. The basement membrane of the basal cells attaches via hemidesmosomes to the underlying Bowman’s layer, while anchoring fibrils pass through the hemidesmosomes to the Bowman’s layer and down into the underlying stroma, where they attach to anchoring plaques of the extracellular matrix, forming an anchoring complex.7 |
• Bowman’s layer. This acellular layer, approximately 8µm to 14µm thick, is composed primarily of collagen and organized as randomly arranged fibrils 20nm to 25nm in diameter. This fibril arrangement evens out towards the posterior as it intermingles with the underlying stroma. Bowman’s layer is highly resistant to penetration or damage; however, if it does become injured, it cannot regenerate, leading to its necessary replacement by epithelial tissue or stromal scar tissue.
• Stroma. The thickness of the cornea is largely due to the presence of the stroma, which measures approximately 500µm thick. This medial layer is composed of an organized network of collagen fibrils and extracellular ground substance, a porous, hydrated gel composed primarily of proteoglycan aggregates. Keratocytes—specialized fibroblasts—is the primary cell type within the stroma and help maintain its integrity. They also produce collagen, glycosaminoglycans (GAGs) and matrix metalloproteinases (MMPs). As such, the precise organization of collagen fibrils within the corneal stroma is imperative to maintaining corneal clarity and appropriate stromal hydration.
• Descemet’s membrane. Measuring just 3µm to 5µm thick, Descemet’s lies between the corneal stroma and the endothelium, essentially functioning as the basement membrane of the corneal endothelium. Descemet’s membrane is separated into the anterior lamina, which is composed of the collagen that gives the layer its elastic properties, and a posterior lamina, which is secreted by the endothelium. The posterior lamina is constantly produced by the endothelium, thickening throughout life. Like Bowman’s layer, Descemet’s membrane is highly resistant to trauma but can regenerate if damaged.
• The corneal endothelium. The posterior-most corneal layer consists of a single polyhedral cell layer that maintains the cornea’s deturgescence via ionic pumps. The cells’ basal surface lies against Descemet’s membrane, while their apical surface lines the anterior chamber. Though the corneal endothelial cells are joined by gap junctions and tight junctions, the barrier formed by the two is more penetrable than the cornea’s anterior surface, thus allowing for corneal uptake of nutrients from the aqueous humor. These cells cannot divide or replicate, so when they are lost, those that remain employ changes in shape or size to fill the spaces in the endothelium.
Epithelial Wound Healing
The process of corneal epithelial wound healing can be divided into phases that occur in sequence, but may overlap in time. They are the latent or lag phase, migration, proliferation and epithelial reattachment.
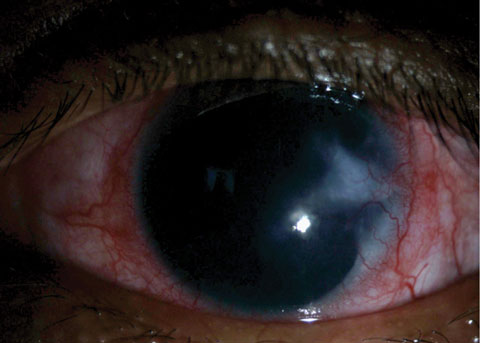 |
| Fig. 2. This patient presented with a vascularized corneal scar. |
• The latent phase. The first phase of the corneal wound healing process is characterized by cellular remodeling and changes to tear composition in preparation for healing.12 This phase results in an increased production of enzymes (including MMP-9s), which degrade the damaged epithelial basement membrane.4 Matrix metalloproteinases decrease cellular adhesion and help enhance cellular migration; they are also important in the degradation and remodeling of normal extracellular matrix (ECM) maintenance. However, excessive levels of these enzymes can hinder healing.13
The latent phase involves several distinct steps and takes place over several hours: first, epithelial cells damaged during injury undergo apoptosis and are shed into the tear film. Next, adherens junctions and gap junctions in cells near the border of the defect are lost and the attachments of basal cells to the basement membrane near the wound edges are broken down. These basal cells then change shape and lose their microvilli, before they form cellular extensions known as filopodia and lamellipodia.9 The epithelial defect is then coated with fibronectin, which serves as a foundation for the adjacent epithelial cells to migrate over.
• Migration. The next phase occurs as cells near the wound edge flatten and spread. The filopodia and lamellipodia are sent out and form temporary attachments to the substrate; contractile elements then pull the cell forward toward the defect.4 Adjacent cells remain attached by desmosomes and maintain their position relative to each other as they slide across the denuded area. The aforementioned temporary attachments are then cleaved, and the filopodia and lamellipodia are again sent forward to repeat the process. This cycle continues until the defect is completely sealed by a single layer of cells. The process typically takes place over 24 to 36 hours, though time can vary depending on the defect’s location and size.
• Proliferation. After migration is complete, the monolayer of cells covering the defect proliferates to restore the normal thickness of the epithelium and fill in the defect. The transient amplifying basal cells reproduce via mitosis and the new cells move inwards toward the center of the defect, then upwards to fill it.9 Cells convert from basal cells to wing cells, then finally squamous surface cells as they move. Tight junctions form to re-establish the cornea’s barrier function, and gap junctions, adherens junctions and desmosomes reform between cells.
• Epithelial reattachment. During the final phase, hemidesmosomes reassemble to firmly attach the epithelial layer to the substrate as anchoring fibrils reattach to anchoring plaques in the stroma. As long as the basement membrane did not receive any damage, this process occurs in a matter of days; if damage did occur however, final formation of attachments and re-anchoring of cells can take months or longer.8
Stromal Healing
The process of stromal remodeling break down resynthesizes and reorganizes the corneal stroma following stromal and epithelial injury. This process, which involves the differentiation and actions taken by keratocytes, is typically lengthy and crucial for restoring corneal transparency.
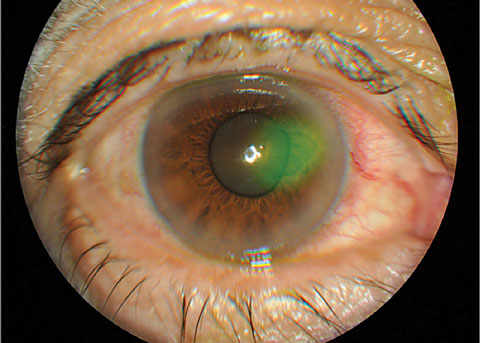 |
| Fig. 3. Traumatic cornea abrasion stained with sodium fluorescein. Click image to enlarge. |
The first stromal event following epithelial injury is keratocyte apoptosis. In this stage, soluble mediators from the corneal epithelium cause cell death via apoptosis of the underlying stromal keratocytes, while other keratocytes undergo transformation into fibroblasts and myofibroblasts. Keratocytes also increase production of the chemokines that attract other inflammatory cells to the corneal stroma from the limbal blood supply and tear film. These inflammatory cells scavenge the remains of apoptotic cells and debris; keratocytes may also eventually become fibroblasts and participate in wound closure as well as play a role in nerve regeneration.13
Myofibroblasts within the corneal stroma are thought to derive from keratocytes via influence from transforming growth factor beta (TGF-β) and platelet-derived growth factor (PDGF). The myofibroblasts lay down a provisional ECM and generate contractile forces in an attempt to close the wound; as such, they are important in collagen and ECM remodeling, as well as in corneal haze formation and regression.14 A delay in regeneration of the epithelial basement membrane (EBM), due to damage, dystrophy or elevated levels of MMP-2 and MMP-9 can allow TGF-β and PDGF to continue entering the corneal stroma from the epithelium, which perpetuates the generation of myofibroblasts.
Ongoing myofibroblast presence can lead to an abundantly disorganized ECM, which contributes to corneal opacity and scarring. Myofibroblasts can also hinder the appropriate restoration of the anterior stromal keratocyte population, which is critical to full recovery of the EBM.6 Only when the EBM is appropriately re-established do proper stromal levels of TGF-β and PDGF settle, causing myofibroblast apoptosis, keratocyte repopulation, clearing of abnormal ECM and the restoration of corneal transparency.
Inhibition of Proper Healing
The corneal epithelium is maintained in a complex balance that can be easily disrupted. Abnormalities in the lids or tear film, damage to corneal nerves, injuries and infections can all compromise corneal integrity.
Epithelial defects are deemed persistent (PEDs) when they remain unresponsive to treatment two weeks after therapeutic initiation. PEDs may result in disassembly of hemidesmosomes and the degradation of Bowman’s layer and the corneal stroma. When corneal healing is impaired, the cornea becomes especially vulnerable to repeated epithelial defects or recurrent erosions, neovascularization or chronic stromal inflammation and scarring (Figure 2).
Recurrent corneal erosions (RCE) may occur following trauma or in certain corneal dystrophies (Figures 3 and 4). They wear away the corneal epithelium due to defective epithelial cell anchoring, which can occur from the improper formation of the anchoring complex, or as a result of abnormalities of the EBM.
Abnormalities in composition and formation of the basement membrane appear to be associated with epithelial basement membrane dystrophy (EBMD) as well as RCE. Corneas displaying EBMD produce redundant layers of basement membrane that can extend into the corneal epithelium instead of lying beneath it.15 These additional layers of basement membrane, seen as lines or fingerprints within the cornea, may prevent the normal movement of cells from the basal layer through to the wing and surface cell layers of the epithelium. Additionally, cells can become trapped with cellular debris in the basement membrane to form cysts. Abnormal cell layering and inadequate adhesion of cells to the underlying stroma may lead to disruptions in corneal clarity as well as painful RCEs. Disruptions in the corneal basement membrane can also lead to increased levels of TGF-β1 and PDGF in the corneal stroma, leading to decreased transparency.16
 |
| Fig. 4. Traumatic cornea abrasion stained with sodium fluorescein. |
Inadequate adhesion of the epithelium to the stroma or injuries that damage the basement membrane and disrupt hemidesmosome formation can lead to RCE, as the poorly adhered epithelium is easily removed via mechanical disruption by the eyelids. Increased levels of MMP-2 and MMP-9 have been found in the tear fluid of patients with RCE.17,18 An increase in MMPs may result in abnormal or excessive degradation of the ECM, hindering proper corneal wound healing and leading to RCEs. Other conditions and lifestyle factors associated with improper corneal healing include diabetes, neurotrophic disease, ocular surface disease and smoking. A study shows that altered cell migration and proliferation signaling pathways, as well as impaired corneal nerve function, is associated with delayed wound healing in diabetic corneas.13
Treatment Advances
Traditional treatment goals for corneal epithelial defects are to minimize pain, decrease the likelihood of infection and expedite healing. A recent increase in understanding of RCE and PED pathophysiology has led to new treatment modalities such as oral tetracyclines, topical steroids, autologous serum eye drops and amniotic membrane (AM) patching or grafting. These treatments aim to decrease inflammation and optimize the environment for healing.
Documented medical use of amniotic membranes (AM) has existed since the early 1900s. The material was used in 1940 by researchers as a biological dressing for the ocular surface and was then rarely mentioned in connection with ophthalmic use again until 1995, when researchers first used it as a surgical graft for ocular surface reconstruction in rabbit corneas. That same year, they used cryopreservation to commercially prepare AM as a graft.11 AM has since experienced a resurgence as an ophthalmic healing modality and is believed to inhibit inflammation, fibrosis and angiogenesis, as well as support wound healing by aiding in cell migration and differentiation. Research also suggests its use as an antimicrobial, antiviral and analgesic agent.
Due to this wide range of reported actions that influence wound healing, AM has been used for the treatment of persistent corneal defects and ulcerations, RCEs, acute chemical or thermal burns, bullous keratopathy, partial limbal stem cell deficiency and in surface reconstruction of the conjunctival tissue.
The amnion, the innermost layer of the fetal placenta, varies in thickness from 0.02 to 0.5mm.20 AM is composed of three basic layers: a single layer of cuboidal epithelial cells; a thick basement membrane layer; and a collagen-rich, nearly avascular mesenchymal layer or stroma. The stroma contains an inner compact layer, middle fibroblast layer and outer spongy layer.21
AM epithelial cells have numerous microvilli and produce cytokines and other factors involved in cell proliferation and differentiation.22-25 They also produce antimicrobial peptides that may decrease the risk of infection.20 The basement membrane (BM) of the AM contains proteoglycans and other molecules that maintain membrane integrity and include various collagens, fibronectin, laminin, fibroblasts and growth factors.11 The BM is used to promote epithelial cell migration, adhesion and differentiation.26
The AM stroma is a collagen rich layer that provides tensile strength and contains abundant proteoglycans, glycoproteins and hyaluronic acid (a glycosaminoglycan). Research demonstrates that the AM stroma suppresses TGF-β signaling, myofibroblast proliferation and differentiation.14 Myofibroblast suppression may help reduce scarring, fibrovascular ingrowth and corneal haze. As such, in this manner AM may modulate healing by promoting tissue reconstruction rather than scar formation.20 The AM stroma also suppresses inflammatory cytokines and sequesters infiltrating inflammatory cells.25,27
A Closer Look: Limbal Epithelial Stem CellsNew corneal epithelial cells are formed in the basal layer, the only mitotically active layer of the cornea.8 Limbal epithelial stem cells (LESC) are located near the limbus and provide a supply of basal cells that later convert into wing and surface cells as they migrate anteriorly. LESCs provide epithelial cells throughout their lifetime and produce transient amplifying cells, which populate the basal epithelium in the peripheral cornea and limbus.10 The cells then migrate centrally, proliferate, and terminally differentiate as central epithelial cells. If the LESC are damaged, corneal epithelial cells may be replaced with conjunctival cells, resulting in opacification of the cornea.11 LESCs generally proliferate slowly but this process is accelerated in response to corneal injury to repopulate the corneal epithelium. |
Research suggests AM may also help maintain nerve growth factor signaling, which is thought to promote nerve regeneration.19,22,25 Additionally, AM may not express most of the major histocompatibility antigens that could result in transplant rejection.19,21,27 More recent studies have found that AM may only produce these antigens in small quantities, which is supported by the lack of significant immune response associated with AM use. This positive property of AM may be related to an immunosuppressive effect exerted by apoptotic AM cells, allowing AM to be used without the need for immunosuppression.11,20,21,23,28
Fresh amnion is a good source of biologically active factors that encourage growth and wound healing, proliferation and migration of epithelial cells as well as ECM remodeling. The tissue can either be heated or air-dried, though doing so may result in loss of some of the tissue’s biological properties. AM may also be lyophilized or freeze-dried, then sterilized by gamma radiation and stored at room temperature. Freeze-dried AM was shown to retain most of its physical, biologic and morphologic characteristics.26 It may also be stored on nitrocellulose paper and preserved in glycerol for cryopreservation. Cryopreserved AM may be stored between 12 to 24 months and may retain more of the membrane’s initial structural, physiological, and biochemical properties.
Research suggests that AM epithelial cells are non-viable in either fresh or preserved preparations, as substances are either released from the devitalized cells or the supporting basement membrane and stroma.3,23,29 Despite this, though some of the biological properties of the preserved AM are retained, they are likely only viable for a limited time.24 Both cryopreserved and dehydrated AM appear to demonstrate comparable clinical efficacy. 26
Amniotic Membrane Grafting
Clinically, AM can be used to treat corneal wounds of a wide range of size and severity. AM may be incorporated as a graft (in the inlay technique), as a bandage (in the onlay technique), or in combination.
During the inlay procedure, the wound is debrided and the AM is applied with its epithelium facing upward, then sutured into place. The AM serves as a permanent substitute for the basement membrane; as such, neighboring recipient epithelial cells eventually migrate onto the AM and integrate it into the host cornea.23 AM can be used as an inlay for patients with PEDs or corneal ulcerations, or after removal of conjunctival lesions.
PEDs or RCEs that are unresponsive to conservative treatments with artificial tears, autologous serum and bandage contact lenses may particularly benefit from an AM, with complete healing generally observed within one to two weeks of its application.31 Research using confocal microscopy to investigate amniotic membrane transplantation (AMT) found that the epithelial layer progressively dissolves over a period of two weeks, though the basement membrane and stroma of the graft were detectable for months.29 In fact, traces of an AM graft can remain for months or years.
As part of the onlay technique, a large amniotic membrane covers the epithelial defect as well as part of the surrounding ocular surface in a fashion similar to a bandage contact lens. It protects the delicate epithelium from the mechanical shearing action of the lids, acts as a barrier to inflammatory cells and substances from the tear film, and aids in hydration of the epithelium. AM orientation may not be particularly important, though placement of the AM stroma-side up may allow for greater contact with the precorneal tear film.19 The AM is sutured to the ocular surface, but detaches in one to two weeks: a fresh AM can be applied if healing is not complete by this point.23 The AM should be changed weekly, If it does remain, it may dissolve over weeks to months, losing efficacy.
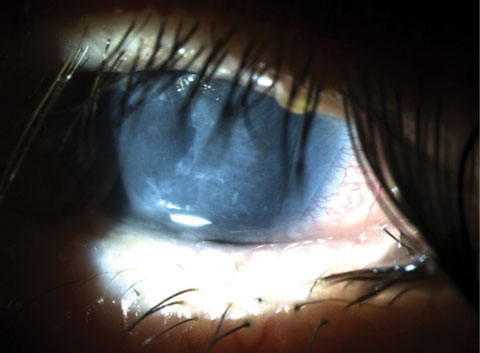 |
| Fig. 5. Sutureless amniotic membrane graft under a bandage soft contact lens for treatment of a neurotrophic ulcer. |
AM can be used as an onlay for conditions characterized by a large amount of inflammation (e.g., following burns or in the case of Stevens-Johnson syndrome), given its anti-inflammatory, anti-angiogenic, anti-scarring and analgesic properties. In these cases, it may be wise to use AM early following disease onset to suppress inflammation and prevent cicatricial complications.
The inlay-onlay technique is used for extensive ulcerations and perforations of the ocular surface. Numerous smaller pieces of AM are used to fill the defect while a larger piece is used as an onlay overtop. The onlay portions protect the inlay pieces and promote epithelialization.
Hands-on VideoTo learn more about the clinical aspects of amniotic membrane application, you can watch this narrated video by Nathan Lighthizer, OD. |
AM may also be used in sutureless techniques along with fibrin glue and either a therapeutic contact lens or a scleral ring conformer (Figure 5).3 Dehydrated AM is available as a sutureless graft, and cryopreserved AM is commercially available in a sutureless form clipped between two polycarbonate carrier rings.
In cases where the LESC population is damaged or deficient, corneal PEDs can occur, or the corneal surface can become populated by the conjunctival epithelium, leading to chronic inflammation, scarring, neovascularization or ulceration of the corneal surface. This leaves the eye vulnerable to perforation or intraocular infection.19 Patients with total LESC deficiency may undergo LESC transplantation using an autograft from the fellow eye if it’s viable, or an allograft from another donor.3,22 AM has also been used as a biological substrate and carrier for culturing of ex vivo limbal epithelial sheets presumably containing LESCs, which research suggests may allow for use of smaller LESC grafts to decrease the likelihood of LESC deficiency for the donor eye.19,23 (See, “A Closer Look: Limbal Epithelial Stem Cells”).
Coding AdviceConfused about how to code and bill for membrane use? Check out this month’s Coding Connection by John Rumpakis, OD, for advice. |
AM grafts are in use for an ever-expanding list of indications. Their positive healing properties, low immunogenicity and biocompatibility make them ideal adjuncts to traditional treatment modalities.
Though AM is anatomically similar to the ocular surface and its physical characteristics as a pliable biological membrane are directly observable, its clinical efficacy is still speculative.31 Widespread anecdotal reports of clinical success suggest that AM shows great potential as an adjunctive modality to the therapeutic armamentarium available to eye care providers to aid in ocular surface healing. However, additional research is needed confirm its efficacy as a first-line modality.
Dr. Tarah Lee is a fellow of the American Academy of Optometry and an adjunct clinical faculty member for the UC Berkeley School of Optometry.
|
1. Dua HS, Faraj LA, Said DG, et al. Human corneal anatomy redefined: a novel pre-Descemet’s layer (Dua’s layer). Ophthalmology. 2013 Sep;120(9):1778-85. 2. Remington L. 2004. Clinical anatomy of the visual system, 2nd edition. Butterworth-Heinemann, St. Louis, MO, 304 pps. 3. Rauz S, Saw VP. Serum eye drops, amniotic membrane and limbal epithelial stem cells--tools in the treatment of ocular surface disease. Cell Tissue Bank. 2010 Feb;11(1):13-27. 4. Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001 Jul;226(7):653-64. 5. Eghrari AO, Riazuddin SA, Gottsch JD. Overview of the Cornea: Structure, Function, and Development. Prog Mol Biol Transl Sci. 2015;134:7-23. 6. Torricelli AA, Singh V, Santhiago MR, et al. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013 Sep 27;54(9):6390-400. 7. Mantelli F, Mauris J, Argüeso P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol. 2013 Oct;13(5):563-8. 8. Ashby BD, Garrett Q, Wilcox MDP. Corneal injuries and wound healing – review of processes and therapies. Austin J Clin Ophthalmol. 2014 March; 1(4):1-25. 9. Steele C. Corneal wound healing: a review. Optometry Today. 1999 Sept; 28-32. 10. Castro-Muñozledo F. Review: corneal epithelial stem cells, their niche and wound healing. Mol Vis. 2013;19:1600-13. 11. Alió JL, Abad M, Scorsetti DH. Preparation, indications and results of human amniotic membrane transplantation for ocular surface disorders. Expert Rev Med Devices. 2005;2(2):153-60. 12. Liu CY, Kao WW. Corneal Epithelial Wound Healing. Prog Mol Biol Transl Sci. 2015;134:61-71. 13. Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015 Nov;49:17-45. 14. Eraslan M, Toker E. Mechanisms of corneal would healing and its modulation following refractive surgery. Marmara Medical Journal. 2009; 22(2); 169-178. 15. Sacchetti M, Macchi I, Tiezzi A, et al. Pathophysiology of Corneal Dystrophies: From Cellular Genetic Alteration to Clinical Findings. J Cell Physiol. 2016 Feb;231(2):261-9. 16. Torricelli AA, Santhanam A, Wu J, et al. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016 Jan;142:110-8. 17. Sakimoto T, Sawa M. Metalloproteinases in corneal diseases: degradation and processing. Cornea. 2012;31 Suppl 1:S50-6. 18. Ramamurthi S, Rahman MQ, Dutton GN, et al. Pathogenesis ,clinical features and management of recurrent corneal erosions. Eye (Lond). 2006 Jun;20(6):635-44. 19. Sippel KC, Ma JJ, Foster CS. Amniotic membrane surgery. Curr Opin Ophthalmol. 2001 Aug;12(4):269-81. 20. Mamede AC, Carvalho MJ, Abrantes AM, et al. Amniotic membrane: from structure and functions to clinical applications. Cell Tissue Res. 2012 Aug;349(2):447-58. 21. Malhotra C, Jain AK. Human amniotic membrane transplantation: Different modalities of its use in ophthalmology. World J Transplant. 2014 Jun 24;4(2):111-21. 22. Tseng SC, Espana EM, Kawakita T, et al. How does amniotic membrane work? Ocul Surf. 2004 Jul;2(3):177-87. 23. Meller D, Pauklin M, Thomasen H, et al. Amniotic membrane transplantation in the human eye. DtschArztebl Int. 2011 Apr;108(14):243-8. 24. Litwiniuk M, Grzela T. Amniotic membrane: new concepts for an old dressing. Wound Repair Regen. 2014 Jul-Aug;22(4):451-6. 25. Dekaris I, Mravicić I, Barisić A, et al. Amniotic membrane transplantation in the treatment of persistent epithelial defect on the corneal graft. CollAntropol. 2010 Apr;34Suppl 2:15-9. 26. Sangwan VS, Burman S, Tejwani S, et al. Amniotic membrane transplantation: a review of current indications in the management of ophthalmic disorders. Indian J Ophthalmol. 2007 Jul-Aug;55(4):251-60. 27. Shimmura S, Shimazaki J, Ohashi Y, et al. Antiinflammatory effects of amniotic membrane transplantation in ocular surface disorders. Cornea. 2001 May;20(4):408-13. 28. Thatte S. Amniotic membrane transplantation: An option for ocular surface disorders. Oman J Ophthalmol. 2011;;4(2):67-72. 29. Nubile M, Dua HS, Lanzini TE, et al. Amniotic membrane transplantation for the management of corneal epithelial defects: an in vivo confocal microscopic study.Br J Ophthalmol. 2008 Jan;92(1):54-60. 30. Clare G, Suleman H, Bunce C, et al. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst Rev. 2012 Sep 12;9:CD009379. 31. Liu J, Sheha H, Fu Y, et al. Update on amniotic membrane transplantation. Expert Rev Ophthalmol. 2010 Oct;5(5):645-661. |

