 Over the last decade, we have become increasingly knowledgeable about the diagnosis and treatment of various ocular surface diseases. In managing these patients, we’ve discovered that some conditions do not respond to topical therapy or other conventional treatments.
Over the last decade, we have become increasingly knowledgeable about the diagnosis and treatment of various ocular surface diseases. In managing these patients, we’ve discovered that some conditions do not respond to topical therapy or other conventional treatments.
However, more advanced procedures, such as amniotic membrane implantation, specifically target and effectively marginalize the underlying causes of advanced ocular surface disease.
Amniotic Membrane
The amniotic sac that surrounds a baby during gestation exhibits incredible anti-inflammatory, anti-scarring and even antimicrobial characteristics.1 The amniotic membrane can be harvested from the mother’s placenta after the normal delivery of a baby.
The tissue primarily is composed of collagen types IV and VII, fibronectin and laminin––some of the most common components of the ocular surface and cornea.2 Further, the amniotic membrane contains hyaluronic acid, which has been found to directly inhibit pro-inflammatory cells and suppress T-cell activation.3 Amniotic membrane also has been shown to stimulate healthy re-epithelialization of damaged ocular surface tissue.4
Clinical Indications
Amniotic membrane implantation isn’t new to eye care. Interestingly, the technique first was used in the 1940s to treat caustic burns of the cornea and conjunctiva, as well as after symblepharon removal.5,6
Currently, the primary indications for amniotic membrane implantation include keratitis (including neurotrophic keratitis), superior limbic keratoconjunctivitis, persistent epithelial defects, chronic non-responsive superficial punctate keratopathy, infectious corneal ulcers, recurrent corneal erosion, toxic keratitis or chemical burns, Salzmann’s nodular degeneration, and even limbal stem cell deficiency.2,6-10
The Procedure
Cryopreserved amniotic membrane grafts, such as ProKera (Bio-Tissue), are stored in a freezer, whereas dehydrated implants can be left at room temperature. Before applying the graft, an anesthetic is administered to the affected eye. Additionally, the graft is lavaged with sterile saline solution to remove any preservatives.
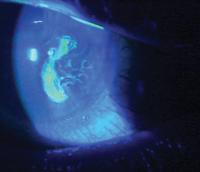
This patient with a persistent epithelial defect and recurrent corneal erosion recently underwent treatment with
ProKera (Bio-Tissue).
First, the graft is inserted under the upper eyelid (while the patient looks down), and then is placed in the lower cul-de-sac (while the patient looks up). Subsequently, surgical tape is applied to the closed eyelids.
Afterward, patients are informed that the less movement they can make with their eyes, the more comfortable the device will be. If the patient has a persistent epithelial defect for example, antibiotics should be prescribed to prevent infection.
Not only is an amniotic membrane implant physically protective like a bandage contact lens, but it can also transfer many of its nutrients to the cornea or ocular surface. In most instances, the graft’s protective effects will persist for seven days. However, in more inflammatory conditions, the amniotic membrane may dissolve within three to four days as its nutrients are transferred and exhausted.
For the first few days after implantation, it is important to see amniotic graft patients daily to provide reassurance and assess healing––especially in those with epithelial defects. Fluorescein dye can be instilled on top of the amniotic membrane ring to monitor re-epithelialization, without the need for removal, until the cornea is fully healed or the membrane is no longer intact.
Once treatment is completed, the ring may be removed. This is achieved by topically anesthetizing the eye, then grabbing the edge of the ring with a forceps while the patient looks down.
Amniotic membrane implantation is a very effective and valuable tool for eye care providers who frequently manage patients with significant ocular surface disease presentations.
Such implants facilitate rapid ocular surface re-epithelialization and have been shown to support the expansion of limbal stem cells, which may further aid in the corneal healing process.11
Dr. Karpecki is a paid consultant to Bio-Tissue Inc. Neither he nor Dr. Shechtman have any direct financial interest in the products mentioned.
1. Talmi YP, Sigler L, Inge E, et al. Antibacterial properties of human amniotic membranes. Placenta. 1991 May-Jun;12(3):285-8.
2. Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004 Jan-Feb;49(1):51-77.
3. He H, Li W, Tseng DY. Biochemical characterization and function of complexes formed by hyaluronan and the heavy chains of inter-alpha-inhibitors. purified from extracts of human amniotic membrane. J Biol Chem. 2009 Jul 24;284(30):20136-46.
4. Pachigolla G. Prasher P, Di Pascuale MA, et al. Evalution of the role of ProKera in the management of ocular surface and orbital disorders. Eye Contact Lens. 2009 Jul;35(4):172-5.
5. Fernandes M, Sridhar MS, Sangwan VS, Rao GN. Amniotic membrane transplantation for ocular surface reconstruction. Cornea. 2005 Aug;24(6):643-53.
6. Gris O, Guell JL, Lopez-Navidad, et al. Application of the amniotic membrane in ocular surface pathology. Ann Transplant. 1999;4(3-4):82-4.
7. Anderson DF, Ellies P, Pires RT, Tseng SC. Amniotic membrane transplantation for partial limbal stem cell deficiency. Br J Ophthalmol. 2001 May;85(5):567-75.
8. Kheirkhah A, Tabatabaei A, Zavareh MK, et al. A controlled study of amniotic membrane transplantation for acute Pseudomonas keratitis. Can J Ophthalmol. 2002 Jun;47(3):305-11
9. Sheha H, Liang L, Li J, et al. Sutureless Amniotic membrane transplantation for severe bacterial keratitis. Cornea. 2009 Dec;28(10):1118-23.
10. Kheirkhah A, Casas V, Raiu VK, Tseng SC. Sutureless amniotic membrane transplantation for partial limbal stem cell deficiency. Am J Ophthalmol. 2008 May;145(5):787-94.
11. Tseng SC, Chen SY, Shen YC, et al. Critical appraisal of ex vivo expansion of human limbal epithelial stem cells. Curr Mol Med. 2010 Dec;10(9):841-50.

