A 45-year-old black female presented with no ocular or visual complaints. Her ocular history was unremarkable. However, her medical history was significant for a recent diagnosis of lupus. Her rheumatologist instructed her to undergo a baseline ocular examination prior to initiating Plaquenil (hydroxychloroquine, Sanofi-Aventis) therapy. What ocular testing should our patient undergo?

Problems with Plaquenil Use
Toxic maculopathy associated with chloroquine use was first documented in the literature five decades ago.1 In the United States, Plaquenil––an analog to chloroquine––is used to treat a variety of conditions, including rheumatoid arthritis, lupus and several distinct inflammatory disorders. Although the incidence of macular toxicity is infrequent with Plaquenil use (at a dosage of 200mg or 400mg q.d.), its visual impact can be devastating.2,3
The associated classic retinal toxicity is described as a bull’s eye maculopathy (ring of depigmented retinal pigment epithelium that spares the foveal area). Initially, central visual acuity may be unaffected, but the patient may notice related paracentral scotomas that often interfere with reading. Eventually, the scotomas will involve the fovea, resulting in deterioration of central vision.
Vision loss typically is permanent, with little significant improvement, and may continue to progress even years after drug cessation.
Previous Plaquenil Testing Guidelines
In 2002, the American Academy of Ophthalmology (AAO) established ocular examination guidelines for screening patients on Plaquenil therapy. Testing included a comprehensive eye exam that consisted of an assessment of the posterior segment with careful evaluation of associated macular changes or signs of retinal disease.4 Baseline fundus photography was considered as an optional test.
Subjective testing of the central visual field was recommended to uncover early functional changes associated with paracentral scotomas. Paracentral scotomas have been reported prior to the associated maculopathy, so an amsler grid test or central 10-2 visual field perimetry was recommended.2,5
Additionally, color vision––helping in the assessment of macular integrity––was recommended as an optional test. There have been reports of color vision defects associated with chloroquinine- and hydroxychloroquinine-related retinal toxicity.4 Other optional tests included multifocal electrophysiology (mfERG) testing and fluorescein angiography.
There are numerous risk factors that promote the development of maculopathy (see “Primary Risk Factors” above). Because both the kidneys and the liver clear the drug, caution needs to be taken in patients with renal or hepatic disease.
In addition, toxic maculopathy has been reported to be dose dependent, whereby retinal toxicity risk increases with more than 6.5mg/kg of body weight.6
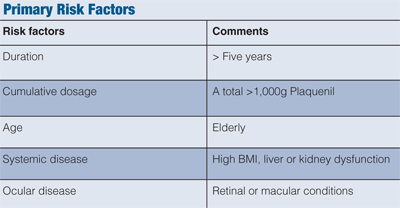
Other risk factors include advanced age and pre-existing retinal disease. Patients with any of the aforementioned findings are considered “high risk” and should be monitored closely.
2011 Testing Guidelines
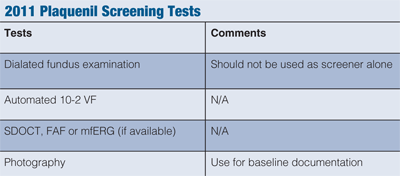
Since the initial screening and monitoring recommendations were published in 2002, there have been reports of retinal toxicity associated with the use of Plaquenil. Furthermore, the advent of novice sensitive diagnostic modalities has led to a revision of the recommended guidelines for patients using Plaquenil (see “2011 Plaquenil Screening Tests”).7
A dilated fundus examination is critical in detecting and documenting established macular or retinal disease; however, due to low sensitivity, it should not be used as a screening test alone. Although fundus photography can be used for baseline documentation of retinal disease or to evaluate apparent subsequent changes, it is not sensitive enough to identify early findings associated with retinal toxicity. So, although baseline photography should be considered, photography is not used as a screening marker.
It is known that color vision defects are not pathognomonic to retinal toxicity and are often observed in a number of macular and optic nerve diseases. Additionally, amsler grid has shown to lack sensitivity to detect early defects and remains a highly subjective test. Due to the disagreement of specificity and sensitivity of these two tests, both may be considered as adjunctive to the examinations, but are no longer recommended for screening. The 10-2 visual field test continues to be recommended as a functional assessment of macular damage.
The evaluation of more recent sensitive diagnostic modalities has shown that objective documentation of structural and functional change can aid in the assessment of early damage and can further quantify such damage.8,9 When available, it is now recommended that routine testing include one of the following: spectral domain optical coherence tomography (SD-OCT), mfERG or fundus autofluorence (FAF). Early changes in the SD-OCT may reflect localized areas of macular thinning in addition to disruption at the photoreception integrity line or inner/outer segment layer. In regards to the FAF, early damage associated with localized areas of RPE defects are characterized as a reduction of autofluorescence, while photoreceptor damage is associated with an increase of autofluorescence. Multifocal electrophysiology can objectively help document any associated localized paracentral functional damage. Using numerous tests becomes more important if the patient is in the higher risk category.
Baseline testing should be initiated within the first year drug therapy in order to rule out any pre-existing retinal condition in addition to establishing documentation of structural and functional ocular status. Baseline data may also uncover high-risk patients. A sharp increase in the prevalence of retinal toxicity is noted following five to seven years of medication use.6 Newer literature emphasizes the risk of cumulative dosing that occurs over a period of time (>1,000g) rather than the previous suggestion of 6.5mg/kg of body weight. Thus, annual screening is recommended after five years for non-high risk patients.
Remember, any patient who is at a high risk for Plaquenil toxicity should be examined on an annual basis following the baseline exam. The goal of monitoring/screening is to identify early macular damage prior to irreversible vision loss or even before visible signs of bull’s eye maculopathy. And although the current guidelines do not discuss the importance of continuous ocular care with an annual comprehensive eye exam, we believe that it is an essential consideration.
1. Hobbs HE, Sorby A, Freedman A. Retinopathy following chloroquinine therapy. Lancet. 1959 Oct 3;2(7101):478-80.
2. Elman A, Gullberg R Nilsson E, et al. Chloroquine retinopathy in patients with rheumatoid arthritis. Scand J Rheumatol. 1976;5(3):161-66.
3. Levy GD, Munz SJ, Paschal J, et al. Incidence of hydrochloroquinine retinopathy in a large multicentered outpatient practice. Arthritis Rheum. 1997 Aug;40(8):1482-6.
4. Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002 Jul;109(7):1377-82.
5. Payne JF, Hubbard GB 3rd, Aaberg TM Sr, Yan J. Clinical characteristics of hydroxychloroquinine retinopathy. Br J Ophthalmol. 2011 Feb;95(2):245-50.
6. Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010 Jun;62(6):775-84.
7. Marmor MF, Kellner U, Lai TY, et al. Revised recommendations on screening for chloroquinine and hydroxychloroquinine retinopathy. Ophthalmology. 2011 Feb;118(2):415-22.
8. Kellner S, Weinitz S, Kellner U. Spectral domain optical coherence tomography detects early stages of chloroquine retinopathy similar to multifocal electroretinography, fundus autofluorescence and near-infrared autofluorescence. Br J Ophthalmol. 2009 Nov;93(11):1444-7.
9. Lai TY, Ngai JW, Chan WM, Lam DS. Visual field and multifocal electroretinography and their correlations in patients on hydroxychloroquine therapy. Doc Ophthalmol. 2006 May;112(3):177-87.

