Ophthalmic practitioners have an arsenal of instruments and procedures available to them to aid in the diagnosis and treatment of ocular pathologies. The principal players in the diagnosis of retinal diseases include intravenous fluorescein angiography (IVFA) and optical coherence tomography (OCT). Although some have suggested that the advancement of OCT technology will render IVFA obsolete, both of these tools have uniquely useful capabilities, allowing each to remain relevant and necessary in the eye care arena.
IVFA: How It Works
Traditional IVFA utilizes the inert dye sodium fluorescein to image the choroidal and retinal vasculature.1-7 First synthesized in 1871 by Von Baeyer, an injectable form was not available for use until the 1930s. In 1961, the first ocular photos were taken, demonstrating IVFA as a useful tool for assisting in the treatment of ophthalmic disease.7-11 The dye’s properties make it highly fluorescent when activated by short wavelength light, with a peak absorption in the blue visible spectrum (485nm to 500nm) and fluorescence occurring in the yellow-green spectrum (520nm to 530nm).7-11
In most circumstances, 5mL of 10% fluorescein are rapidly infused via a butterfly needle into the antecubital vein of the elbow or radial vein of the wrist.9,11 Though rapid infusion may provide a better angiogram, it also can increase the incidence of adverse reaction.7,11 In general, IVFA is well tolerated, but adverse reactions occur in 5% of cases.7,11-13 Documented adverse reactions range from transient nausea and vomiting, discoloration of the skin, and mucous membranes and urine, to flushing of the skin, pruritis and urticaria.7,11-13 Syncope, extravasations of the dye into surrounding tissues, laryngeal edema, bronchospasm, anaphylaxis, hypotension, seizures, myocardial infarction and cardiac arrest have been documented.7,9,10-13 One death in 220,000 patients who received IVFA also has been reported.11,13 Contraindications to IVFA include known hypersensitivity to the chemical and pregnancy.7,11,13 Nursing mothers are discouraged from breast feeding for at least 24 to 48 hours after angiography.7,13 Oral fluorescein has been developed as an alternative to injection for squeamish and young patients but is not the method of first choice.8,11,13
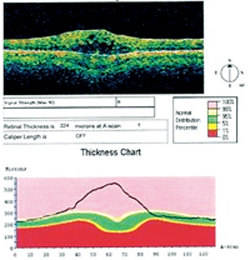
OCT image depicting changes from CME.
Color and red-free fundus photographs are usually obtained.9,11 To capture the IVFA on film, a fundus camera is equipped with a blue excitation filter (490nm), which changes the flashlight to the correct excitation wavelength. A yellow-green barrier filter (525nm) limits the light that is returning to the film plane of the camera to the exclusive wavelength of the excited dye.7,8,10 The blue light enters the retina and “excites” the circulating fluorescein. The light given off by the energized dye leaves the eye and encounters the film plane of the camera through the barrier filter, which only allows the excited yellow-green light to be recorded onto film.7,8,10
Photos are usually taken at four-second intervals, beginning 15 seconds prior to the injection and continuing with a tapered frequency for 10 to 20 minutes.9,11 The timing of the photographs and exposure interval is adjusted frequently, based upon the ocular and medical situation. For example, in cases where choroidal neovascularization is suspected, the majority of photographs typically are taken at the beginning of the test and with greater rapidity, because these vascular malformations begin to leak profusely in the choroidal flush phase.
In cases of diabetic clinically significant macular edema (CSME), the capillary leakage is slow and not apparent until later phases, dictating a strategy of a spread out sequence with tighter frequency in the middle. Cystoid macular edema (CME) often is not apparent for five to 10 minutes after the start of the test, so these studies are adapted to place emphasis on capturing more photographs of the recirculation phase.
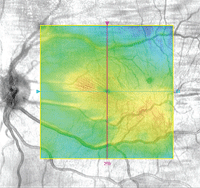
OCT image depicting macular pucker.
Once the fluorescein is injected, approximately 60% to 80% binds to serum proteins (e.g., albumin). The rest remains unbound in the circulation as free fluorescein.7-9 This, along with the configuration of the ocular anatomy, is what generates the presence and absence of fluorescein on the angiogram. The retina has both inner and outer blood-retinal barriers which, when intact, prevent the leakage of blood products.9,11 The tight junctions of the unfenestrated retinal capillary endothelial cells make up the inner blood-retinal barrier.
The outer blood-retinal barrier is formed as a result of the tight junctional complexes between adjacent retinal pigmented epithelial (RPE) cells.9,11 The former prevents passage of fluorescein into extravascular spaces, and the latter prevents fluorescein from crossing from the choroid into the neurosensory retina.9,11 In contrast, the choriocapillaris consists of fenestrated, thin-walled blood vessels that allow the passage of the unbound fluorescein into the extracellular spaces and across Bruch’s membrane.8-10
Five Phases of a Fluorescein Angiogram
The first phase is known as the pre-arterial phase or choroidal flush phase. Fluorescein flows from the injection site to the heart and then, is pushed into the systemic vascular tree. The time it takes for the dye to reach the eye via this pathway is known as transit time. The dye moves into the eye via the internal carotid system. As the dye moves downstream into the ophthalmic artery, it transitions into the long and short posterior ciliary arteries and into the fenestrated capillary network of the choriocapillaris. The fenestrations allow fluorescein to mingle in the choroid, illuminating the entire vascular watershed. This defines the choroidal flush. The pre-arterial phase in a normal subject begins approximately 10 to 12 seconds post-injection. Increased transit times suggest vascular impediments to efficient blood flow. The dye simultaneously works its way into the central retinal artery and retinal circulation.7-9,11,13 The path to the choroid is slightly shorter than the path into the retina, with a greater blood supply; therefore, the choroid fills about one second before the retinal vessels.7-9, 11,13 If a cilioretinal artery is present, it also will fill at this time since it is supplied by the short posterior ciliary circulation.7
The rapid influx of fluorescein into the choroidal anatomy, along with the variability of the overlying RPE, causes the choroid to take on a mottled or “ground glass” appearance.7-9,11,13 There is no filling of fluorescein in the macular area secondary to the presence of yellow xanthophyll pigment, lipofuscin deposition, avascularity at the foveal center and an increased presence of melanin in the RPE of the fovea.7,9
The second phase is known as the arterial phase. Here, the arterioles sequentially become filled with fluorescein, while the venules remain unfilled. The arterial phase begins just seconds after the choroidal flush phase, with almost simultaneous filling of the entire arterial tree.7-9,11,13
Next, the arterial-venous (AV) phrase represents the earliest detectable changeover of flow from the arterial retinal capillaries into the retinal venules. This phase is marked by laminar flow or the “railroad track” appearance in the retinal venules, which results from the fluorescein entering the venules at their sides. The blood flow in contact with the lumen of the vessels is moving more slowly than the blood in the middle of the vessel, secondary to increased friction.
As the transition occurs, this causes the blood positioned along the sides of the venules to fluoresce—first creating the pathognomonic appearance of a locomotive track. The vascular property is known as laminar flow.7-9,11,13
The fourth phase is the venous phase. Here, there is complete venous filling with the beginnings of venous drainage from the eye. The venous phase is marked by complete filling of the veins, usually within 30 to 35 seconds of injection.7-9,11,13
The fifth phase is the late or recirculation phase. Here, the clinician may observe second and third passes of the dye both into and out of the eye. In this phase, the fluorescence is greater in the venules than the arterioles.7-9,11,13
Interpretation of IVFA
Interpretation is based upon the findings of hypofluorescence or hyperfluorescence. Hypofluorescence occurs by one of two mechanisms:7-9,11
- The view of the dye is blocked secondary to an obstruction, such as blood or pigment present in the retina itself.
- The blood/dye is not flowing into the tissue or an area (nonperfusion).
Intraretinal bleeding, RPE hyperplasia and hypertrophy, subretinal or choroidal neovascular membranes (CNVM) that extensively leak blood and serosanguinous fluid into the retina or choroid are among the primary causes of blockage defects.7-9,11 Intraretinal and preretinal barriers, such as vitreous hemorrhage, dense cotton-wool infarcts and medullation of nerve fibers, also can prevent the transmission of the fluorescence.7-9,11 Hypofluorescence, due to impaired vascular filling, can occur in three areas—the choroid, retina or optic nerve.7-9,11
Clinically, the differentiation between intraretinal and subretinal etiologies is based upon the appearance of the underlying scleral tissue or overlying vessels.7-9,11,14 The optic nerve also may demonstrate a lack of normal fluorescence secondary to tissue loss (e.g., optic pit) or capillary non-perfusion as a result of optic atrophy or anterior ischemic optic neuropathy (AION).7-9,11,14
Increased areas of fluorescence are known as hyperfluorescence. The three main etiologies of hyperfluorescence include:7-9,11
- Leakage of the dye from defective blood vessels.
- Accumulation of the dye in abnormal spaces.
- Ocular tissues permitting increased visibility of the dye as seen in defects of the RPE.
Leakage is found when there is a breakdown of the blood-retinal barrier. This includes pathologies that can produce intraretinal microaneurysms, telangiectasia and neovascularization.7-9,11,13,14 Here, fluorescein oozes from the compromised vasculature, becoming visible as fluorescence that increases in size and intensity over the course of the angiographic sequence.
Fluorescein also can pool in the spaces that are created following sub-RPE or sub-sensory retinal detachment. This can include pathologies, such as RPE detachment and idiopathic central serous chorioretinopathy (ICSC).7-9,11 Sometimes the sclera, optic nerve, drusen and glial tissue will stain in the late phases of the test, creating a hyperfluorescent appearance.7-9,11 Chorioretinal scars, angioid streaks, lacquer cracks, geographic atrophy and full-thickness macular holes are among other conditions that hyperfluoresce due to the loss of overlying tissues and increased visibility of the fluorescein in the tissues below.7-9,11,13,14
Indications for IVFA include (but are not limited to): the treatment of diabetic retinopathy, age-related macular degeneration with subretinal neovascular membranes, central and branch retinal artery and vein occlusions, ICSC, CME, retinal arterial macroaneurysms, CNVM associated with pattern dystrophies of the RPE, choroidal tumors, CNVM associated with choroidal inflammatory conditions, and hereditary retinal dystrophies.7,11 The test permits the visualization of leakage that is treatable with laser photocoagulation. It also enables the treating surgeon to discover landmarks that help in aiming the laser.
Indocyanine Green Angiography
While IVFA is most useful in diseases that involve the retina, it is limited in the treatment of hemorrhagic retinal or subretinal disease because a more detailed view of the structures is required for both diagnosis and treatment. Sodium fluorescein is a relatively small molecule whose observation is camouflaged in the presence of intraretinal or preretinal blood and whose properties make it incapable of remaining within the choroidal vasculature. Therefore, many disease entities cannot be discerned to allow the initiation of therapeutic treatment.
A study that may augment the treatment of subretinal pathologies is indocyanine green angiography (ICGA).14-24 Indocyanine green (ICG) dye has two major differences when compared to sodium fluorescein that make it better suited to image the choroidal vasculature:
- Its excitation and emission peak at 800nm to 810nm and 835nm respectively in the near-infrared spectrum, allowing for better penetration of viewing through pigments such as xanthophyll, hemoglobin and melanin.1-6,8,9,14,25
- ICG demonstrates 98% plasma protein binding (mostly albumin) that allows it to remain mainly intravascular, even as the dye circulates through the fenestrated capillaries of the choriocapillaris.1-6, 9,25
ICG is a well-tolerated, tricarbocyanine dye, with a molecular weight of 775 daltons that absorbs light at 790nm to 805nm and has a peak emission at 835nm. While the substance yields just 4% of the total fluorescence of sodium fluorescein, its spectral properties allow it to be visualized through ocular pigments, blood and serous fluids.2,8,9 Because ICG requires light in the infrared spectrum and has minimal fluorescent properties, it requires more sophisticated equipment than a standard camera and film to capture the angiographic images.
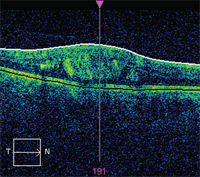
OCT image exhibiting areas of intraretinal fluid in a case of CSME.
The scanning laser ophthalmoscope (SLO) and digital capturing systems have mainstreamed image capture, improved image resolution and increased image contrast to provide a system for better visualization of pathology.1,3-6 ICG, which has a high affinity for serum albumin, is excreted through the biliary system rather than the kidneys.3,5 ICG contains 5% iodine by weight, and patients with potential hypersensitivities should be pre-screened for iodine allergy and seafood allergies to avoid cross-sensitivity.1,3,5,8,9
Overall, ICGA is tolerated better than IVFA, with documented adverse reactions including (but not limited to): vomiting, itching, urticaria, syncope, transient nausea and anaphylaxis.1,3,8 Local extravasation of the ICG produces minimal damage in contrast to IVFA, where tissue necrosis can occur.1,5
Optical Coherence Tomography
As opposed to the methods that allow direct visualization of the vascular tree, OCT enables direct, noninvasive observation of retinal tissues in cross section.
The use of OCT to obtain noninvasive structural images of ocular structures has been incorporated into diagnostic equipment since the early 1980s. Today, OCT technology is capable of providing real-time, high-resolution images of ocular tissues. Optical tomography utilizes diffraction tomography, diffuse optical tomography and OCT.26 These approaches have been integrated into technologies that provide structural diagnostic information of the retina proper, retinal nerve fiber layer, optic nerve head, cornea and anterior chamber.27
Unlike other tomographic technologies, OCT provides depth resolution. The depth resolution is not bound by transverse or axial resolution, allowing for advantageous application toward ocular scans inside the limited aperture of the pupil. In addition, OCT also provides a relatively high scanning depth within scattering media. It does so in a contact-free manner.27,28 OCT is well-suited for imaging translucent biological structures, including vasculature and soft tissue.29
Light from a focused coherent point, known as the source beam, is directed into the pupil through a generic beam splitter. A portion of this light is known as the reference beam. It is directed toward a reference mirror by the beam splitter. The remaining portion of the coherent beam that is directed into the pupil and reflected by the incident ocular tissue is known as the sampling beam. Both the reference beam and the sampling beam return to the beam splitter and are simultaneously reflected, creating the detector beam.
The detector beam is captured by a photo detector. The photo detector measures the relative difference in time or change in wavelength between the respective beams. The relative position of the plane of the beam splitter compared to the plane of the ocular tissue can be adjusted to provide a transverse scanning pattern. The distance between the beam splitter and the reference mirror can be adjusted to provide a structural baseline for tomographic data. Then, this raw data is compiled and interpreted with a computer and integrated software to create various dimensional scans of the selected tissue.26,27
Biological tissue, including tissue within ocular structures, is not homogenous in nature. Individual cells are suspended within an extracellular matrix, comprised of various structural proteins, and are packed within diffuse hydrated gels. These irregularities result in almost constant changes in the reflection, absorption and scattering of the sampling beam as it passes through the ocular tissue. The scanning beams range from 600nm to 1300nm for optical tomography. Super luminescent diodes (SLDs) commonly are utilized as the source beams for OCT technologies.26,27 This ensures an adequate light source that can be utilized for high-resolution scans, while maintaining a high safety margin for light directed at in vivo ocular tissues.28
TD-OCT and SD-OCT
Currently, there are two distinct platforms of OCT commercially available for diagnostic and structural imaging of ocular structures.26,30,31 The older platform utilizes a time-domain (TD) strategy for the capture of tomographic data. Time domain refers to the analysis of time delay between the reference beam and the sampling beam, the photo detector and microprocessor.26 A typical TD-OCT provides an axial resolution of approximately 10µm and a transverse resolution of 20µm, with a scan velocity of 40 axial scans per second.28,30
Spectral domain (SD) refers to the analysis of change in wave properties between the reference beam and the sampling beam.26 SD-OCT platforms incorporate an analysis of the detector beam using a Fourier transformation algorithm. The Fourier transformation refers to a complex mathematical construct.30 In the SD-OCT platform, only the transverse component is directly detected. The axial component of the scan is determined by an inverse Fourier analysis of the spectrum of reflected light from the sampling beam.26 The incorporation of this mathematical analysis allows the measurement of all source beam reflections simultaneously.
A typical SD-OCT unit provides an axial resolution of approximately 5µm and a transverse resolution of approximately 10µm, with a scan velocity of 27,000 axial scans per second.30 A significant increase in the amount of data acquired in a typical scan for the SD-OCT platform compared to the TD-OCT platform results in a correlating reduction in eye movement-related artifacts and increased signal-to-noise ratios.31 The increased resolution afforded to the SD-OCT platform allows improved visualization of relatively thin or poorly backscattering structures, such as the external limiting membrane, photoreceptor inner segment/outer segment junction and Bruch’s membrane.29 These structures, in comparison, are poorly delineated using the TD platform.31
The macular thickness quantification logarithm of the Stratus OCT (Carl Zeiss Meditec), a TD platform, utilizes the apparent position of the internal limiting membrane and the photoreceptor inner segment/outer segment junction as the axial boundaries of the retina. The macular thickness logarithm of the Cirrus HD-OCT (Carl Zeiss Meditec), a SD platform, utilizes the apparent position of the internal limiting membrane and the outer band of the RPE as the axial boundaries of the retina. This difference in resolution and subsequent choice of retinal boundary results in an increase in macular thickness of approximately 43µm using the Cirrus OCT compared to the Stratus OCT.31 For this reason, quantification reports cannot be interchanged between TD and SD platforms for monitoring macular thickness.29,31
How OCT Works
The OCT develops a structural cross section of ocular tissues through the combination of individual focal depth scans. Multiple focal scans are combined in a transverse fashion to form a two-dimensional cross section. These scans can then be compiled longitudinally to provide an overall scan of the tissue (retina or optic nerve).28
The scanning images of the OCT can be compared to a deck of playing cards placed on their edge. The deck of cards represents the overall OCT scanning data, with each individual card representing an individual OCT image that is then removed from the deck for viewing. Each playing card––an OCT image––correlates to a specific plane within the scanned ocular structure. Depending on the resolution and computing capacity of the OCT platform, a 3-D representation of the scanned structure also can be extrapolated from the combination of conventional transverse scans.
Individual scanning data may be combined through the use of analytical software to provide a more global representation of a given ocular structure.26,29-31 The representations provide macular and optic nerve fiber layer thickness analysis. Depending upon the given OCT platform, various software strategies can be used to provide epidemiological and statistical analysis of selected scan data.28-31
The OCT scanning patterns may be selected in either a linear or circular configuration.28 A linear scanning pattern will provide a series of scans across a given area of interest. A circular scanning pattern will provide a rotating series of scans centered upon a given focal point of interest. The circular scanning pattern often is selected within TD-OCT platforms to increase the likelihood of obtaining an individual scan through a focal area of interest.
The relative decrease in spacing between individual transverse scans in the SD-OCT provides increased resolution, making a linear scan preferred.30,31 The selected individual OCT image, regardless of platform, includes a directional indicator correlating to the geometric orientation and direction of the scan. Thus, the viewer can be assured of the orientation of the individual scan within the given ocular structure.28,30
The OCT quantifies the focal reflectivity of a given ocular tissue. This focal ocular reflectivity is then represented within the cross-sectional OCT image through a correlating artificial color scheme. OCT images can be represented in either color or grayscale.
Depending upon resolution and the individual diagnostic purpose of the scan, both color schemes may be independently advantageous. Higher levels of reflectivity, roughly correlating to higher optical density, are represented by “hotter” colors within the individual scheme such as red or white, respectively. Individual structures that present with this level of reflectivity include the RPE, nerve fiber layer and choriocapillaris. Layers that correlate to more intermediate levels of reflectivity are represented by yellow or light grey, respectively.
These include the inner and outer plexiform layers of the retina. Other ocular tissues that present with reduced reflectivity, such as the inner and outer nuclear layer of the retina, are represented by green or dark grey, respectively. In contrast, structures that provide minimal reflectivity, such as the photoreceptor layer, vitreous, serous exudate or blood, normally will be represented by blue or black.
Ocular anomalies with extreme levels of reflectivity, such as macular or optic nerve head drusen, are represented as “hot” by a red or white color scheme. They also exhibit a “blocking defect”—creating a loss of reflectivity below the anomaly. This optical defect is due to the material’s high absorption value.28-31
Comparing IVFA and OCT
A cross-sectional OCT image can be utilized to quantify the structural integrity of the retina (thickness and topography). By contrast, traditional IVFA can be used for qualitative analysis of retinal vasculature and sensory retinal integrity.29,32-36 Various fluorescein leakage patterns have been correlated to structural deficiencies within the retina as a direct result of ocular and systemic disease.32,33,37
In many cases, histological sampling of affected retinal tissue has proven these analytical correlations.37,38 Until the development of noninvasive scanning technologies such as OCT, the leakage patterns of IVFA could not be definitively correlated to structural deficiencies in real time.29,32
Now, structural deficiencies within the retina can be quantified through the use of OCT, with and without concurrent use of IVFA.33-35 Current commercially available OCT platforms do not provide adequate resolution below the level of the RPE and thus are unable to offer quantitative analysis of choroidal structures. However, research level OCT platforms, such as enhanced depth-imaging, SD-OCT, have been used to provide structural correlation between OCT and ICG angiographic leakage patterns within the choroidal vasculature.29,30,39,40
Cystoid Macular Edema
CME is a relatively common adverse sequella of a variety of systemic and vascular diseases.41,42 IVFA traditionally is used to confirm retinal leakage, once it has been detected by observation. The classic IVFA pattern seen secondary to CME is described as a petalloid or honeycomb pattern of hyperfluorescence that increases in size and intensity over the course of the angiographic time frame. These patterns are thought to represent dye leakage and pooling within cystoid spaces between the layers of the retina.32 Histopathologic studies of the complication have confirmed the presence of cystoid spaces within the retina, with a predilection to the outer plexiform and inner nuclear layers.38
Cross-sectional retinal images of CME using the OCT have demonstrated three basic structural changes: sponge-like retinal thickening, cystoid thickening and serous detachment of the neurosensory retina.
In comparative studies of CME between fluorescein angiograms and OCT images, the petalloid hyperfluorescence and cross-sectional OCT images define cystoid spaces within the outer plexiform layer in the vast majority of cases. Interestingly, some of the petalloid angiographic patterns correlated only to diffuse retinal swelling on OCT analysis.32
The findings support previous electron microscopy studies, which postulated that petalloid macular hyperfluorescence patterns were the result of fluorescein accumulation within edematous Muller cells.32,37 In comparative studies between fluorescein angiogram findings of honeycomb hyperfluorescence and concurrent OCT imaging, the cross-sectional OCT images indicated retinal swelling in the outer plexiform layer as well as cystic changes within the inner nuclear layer.37,41,42
This correlates to previous investigations that speculated honeycomb hyperfluorescence patterns were the result of vertical fibers within the external plexiform layer and the oblique orientation of these fibers within the foveal region.37,41,42 The OCT is not an alternative to IVFA. Instead, it adds a noninvasive capability for diagnosing and monitoring macular edema. In cases that require treatment, IVFA localizes the landmark positions where the laser should be placed.29,33,43
Vitreomacular Interface Abnormalities
Changes within the vitreomacular interface have the potential to influence diabetic macular edema. Here, edema may continue despite appropriately guided focal laser treatment.41-43 Defined as epiretinal membrane, macular pucker or focal macular adhesions, vitreomacular interface anomalies (VMIA) may be identified by biomicroscopy in advancing cases but fluorescein angiogram is adequately equipped to provide early detection. Cross-sectional OCT images with high-resolution scans on SD platforms have been found to be superior in identifying VMIA.
In cases where edema is being provoked by vitreomacular traction, surgical or pharmacological treatments may provide more efficacious results compared to focal laser treatment alone.44 The use of OCT imaging may facilitate the identification of VMIA and aid in the treatment selection process, providing detailed data in the stratification of patient subgroups.45 Additionally, OCT imaging has been shown to identify focal neurosensory detachments associated with vitreomacular traction in the absence of IVFA findings.29,35,43
Diabetic Macular Edema
In cases of diabetic macular edema, successful treatment—which results in the reduction or resolution of intraretinal swelling—may not necessarily result in increased visual acuity.46 Studies demonstrate that central macular thickness and visual acuity are only modestly correlated in post-treatment cases of macular edema.
These findings indicate that reduced visual acuity is a multifactoral result based on damage or disruption to the retinal architecture, including direct photoreceptor damage rather than reversible intraretinal swelling.41,46,47 SD-OCT has the capability to resolve the inner segment/outer segment junction within the neurosensory retina.29-31,42 Although large disruptions of this junction are identifiable, subtle changes in this segment junction cannot be resolved using TD-OCT platforms. The inner segment/outer segment junction appears as a hyper-reflective line just anterior to the level of the RPE. This junction is known as the photoreceptor interface or integrity line (PIL).
Disruption of the PIL has been correlated to both poor visual acuity and outcome in a number of various retinal pathologies.42,47,48 Traditional IVFA does not possess the ability to discern subtle changes in retinal architecture beyond that identified with fluid leakage.29,32,47 OCT imaging may be used not only to identify pathological changes at an earlier stage than IVFA, but also to indicate potential visual outcome.42,46,47 However, IVFA remains necessary for focal laser application as its telltale leakage serves as a marker for laser application.
Venous Occlusion
Macular edema also is a common, vision-threatening complication of branch and central retinal vein occlusion.33,34,47 Fluorescein angiographic patterns that indicate diffuse or cystoid edema associated with retinal vein occlusion correlate well to the sponge-like and cystoid changes observed in cross-sectional OCT imaging.33,47,49 The association between the central retinal thickness and visual acuity in cases involving retinal occlusion is only mild.46,47 This reduced association is due, in part, to the potential ischemic effects of vein occlusion of the neurosensory retina.47
As with diabetic macular edema, in cases of both branch and central vein occlusion, integrity of the photoreceptor inner segment/outer segment junction is closely associated with visual acuity. IVFA is advantageous in these cases as it maintains the ability to identify areas of retinal ischemia (hypoperfusion/non-perfusion) which cannot be acutely identified using cross-sectional OCT imaging.33,47 Reduced areas of central retinal thickness indicated on OCT image analysis correlate strongly to areas of macular ischemia identified by IVFA.47 Histological studies of areas of retinal capillary non-perfusion as a result of vein occlusion have demonstrated atrophic loss of inner plexiform and inner nuclear layers.38 This correlates not only to reduced retinal thickness in early- and late-stage OCT imaging analysis but also with observed loss of inner retinal layers upon cross-sectional OCT imaging.47
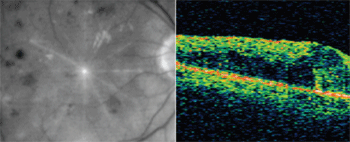
OCT images from patient with venous occlusion and resulting macular edema.
While OCT is an excellent noninvasive study for monitoring progress and detecting macular edema, it is not ideal for identifying areas of non-perfusion or localizing where the laser therapy should be applied.
Idiopathic Central Serous Chorioretinopathy
Idiopathic central serous chorioretinopathy (ICSC) is considered a multifactoral retinal disease where focal defects within the RPE adjacent to the macula become compromised permitting fluid to accumulate within the subretinal space.39,50 Individuals are symptomatic in the early stages. Focal disruptions of the RPE may result from RPE detachments in chronic or recurrent forms of the disease.
Focal areas of leakage from the RPE leading to neurosensory retinal detachment are revealed with IVFA as expanding areas of hyperfluorescence over the course of angiographic frames that exhibit the characteristic “smoke stack or ink blot” appearance. Chronic cases of ICSC are represented by broad areas of hyperfluorescence associated with multiple indistinct areas of leakage on IVFA.39
Observational analysis of the condition has proposed choriocapillaris hyperpermeability secondary to hypertension or steroid usage as the underlying cause.51 Later angiographic studies using ICG confirm this postulate through the appearance of multifocal areas of choroidal vascular hyperpermeability.52
Conventional OCT imaging, both TD and SD platforms, reveals specific cross-sectional findings associated with the various stages of ICSC. Imaged symptomatic cases of ICSC reveal distinct broadened areas of neurosensory retinal detachment with minimal alterations in retinal thickness. These cross-sectional samples may or may not reveal alterations of the foveal pit area.39,50 Focal areas of RPE distortion or “bulges” can be visualized on OCT correlating to focal areas of hyperfluorescence on concurrent IVFA.
Although these focal areas of RPE distortion have been documented through biomicroscopy, OCT imaging confirms that these areas are not in fact RPE detachments but rather areas of disruption associated with increases in choroidal hydrostatic pressure.50 OCT imaging in advanced cases of ICSC reveal distinct largely symmetrical areas of RPE vaulting as well as neurosensory retinal detachment.
These areas of RPE vaulting correlate to the location of the characteristic “smoke stacks” apparent on concurrent IVFA. Neurosensory retinal thinning also tends to accompany these RPE detachments on OCT imaging, indicating secondary retinal pathology.50 ICGA indicates a variety of vascular defects in cases of ICSC including variable areas of filling within the choroid, choroidal venous dilation and choroidal hyperpermeability.39
Enhanced depth imaging OCT (EDI-OCT) has the potential to visualize tissues below the level of the RPE compared to conventional TD and SD platforms. EDI-OCT imaging of the choroid reveals significant increases in thickness of the choroid in cases of ICSC compared to normals.39,40 EDI-OCT also has been used to potentially determine the differing underlying mechanism involved in current treatment options for chronic or recurrent ICSC.
Conventional laser photocoagulation, a standard treatment for classic focal ICSC, may result in reduced leakage on IVFA but does not correlate to a reduction of choroidal thickening on OCT cross-sectional imaging.
Treatment by photodynamic therapy, used primarily in cases of chronic or diffuse ICSC, however, did correlate with reduced choroidal thickening on OCT imaging as well as decreased hyperpermeability on ICGA.39 In cases such as these, where laser therapy is not indicated in the acute phases, OCT imaging may be sufficient as it has the capability to demonstrate images of the pathology that can be accurately interpreted without the risk of IVFA.
Age-Related Macular Degeneration
Exudative age-related macular degeneration (AMD) is a retinal pathology that can result in sudden and irreversible reductions in visual acuity as a result of retinal architecture disruption and scarring, secondary to choroidal neovascularization (CNV) and leakage.36,53,54 Current treatments for CNV include laser photocoagulation, photodynamic therapy and intravitreous vascular endothelial growth factor (VEGF) inhibitors.33,36,53,54 In cases where CNV is suspected, IVFA can be used to demonstrate increasing choroidal hyperfluorescence with late staining.
On OCT cross-sectional imaging, these same areas are imaged as RPE disruption and cystoid retinal edema. Retinal thickness and retinal volume analysis of OCT imaging correlate well to areas of increasing fluorescein leakage.35,48,55,56 In addition, retinal drusen can be visualized on OCT and are identified by their distinctive opaque protruding appearance under the RPE.57
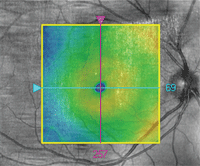
OCT image overlay in a patient with ICSC.
Discrepancies between OCT retinal thickness analysis and IVFA in cases of exudative AMD may be attributable to algorithmic failures associated with the chosen endpoints of the retinal boundaries during OCT scan analysis. In general, TD-OCT platforms have an increased probability of failure to detect subtle increases in leakage that were demonstrated by concurrent IVFA.35,56,57
By contrast, SD-OCT platforms provide a higher sensitivity to structural changes associated with CNV leakage.56 Treatment involving VEGF inhibitors may require monthly monitoring even after stabilization of acute leakage and CNV membrane regression. The noninvasive nature and consistent reliability of OCT imaging presents significant safety and cost advantages compared to IVFA in cases requiring a recurring monitoring and re-treatment regimen.36,53,54
Final Score
OCT technology can produce real-time in vivo cross-sectional imaging of ocular structures and retinal architecture.26-36,39-50,52-57 These images provide both the researcher and clinician with the ability to identify and monitor structural changes associated with retinal pathology that were previously only identifiable by post-mortem histology.32,37,38,47 Having interpretational experience in OCT cross-sectional scans and retinal thickness analysis can aid the doctor in both the diagnosis and treatment of various retinal pathologies.28-36,39-50,52-57 Traditional angiography, both fluorescein and ICG, still maintains functional viability which currently eludes commercially available OCT technology—including the ability to visualize ocular vasculature, blood flow, vasculature leakage and overall perfusion.
Angiography provides information regarding vascular integrity, with its data explaining structural changes, while OCT provides information regarding structural changes. Both diagnostic techniques are superior to the other in specific pathologies and situations. Both are complementary in diagnosis and treatment plan selection in a variety of retinal pathologies.32,33,36,43,47,53,56 However, OCT has a distinct advantage from a safety perspective, given its noninvasive nature, and from a long-term financial perspective because it can be performed by a technician level operator.28,54
OCT has made examination of structure for identifying, confirming or monitoring pathology noninvasive.33,36,53 Research-level OCT platforms, such as Doppler Analysis OCT, may provide the ability to independently visualize vasculature and blood flow at a level of sensitivity currently possessed only by traditional angiography.26,27 Commercial platforms that directly integrate angiography, autofluorescence and OCT technologies are being developed to complement and exploit the summation principal of their individual advantages.29
Dr. Hutchinson practices privately in St. Louis and is an adjunct faculty member at the University of Missouri-St. Louis College of Optometry. Dr. Street is in residency at the Pennsylvania College of Optometry (PCO) at Salus University in Elkins Park, Pa. Dr. Gurwood is a professor at PCO. Dr. Myers is the senior staff optometrist at the Coatesville VA Medical Center in Pennsylvania.
1. Slakter JS, Yannuzzi LA, Guyer DR, et al. Indocyanine-green angiography. Curr Opin Ophthalmol. 1995 June;6(3):25-32.
2. Schneider U, Sherif-Adel S, Gelisken F, Kreissig I. Indocyanine green angiography and transmission defects. Acta Ophthalmol Scand. 1997 Dec; 75(6):653-6.
3. Stanga PE, Lim JI, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003 Jan;110(1):15-21.
4. Bischoff PM, Niederberger HJ, Török B, Speiser P. Simultaneous indocyanine green and fluorescein angiography. Retina. 1995;15(2):91-9.
5. Kumar A, Nainiwal S, Prakash G. Indocyanine green angiography in age-related macular degeneration. Bombay Hosp J. 2002 July;44(3):333-9.
6. Nagpal M, Banait S. Scanning laser opthalmoscopy in macular degeneration. Bombay Hosp J. 2002 July;44(3):340-50.
7. Bennett TJ. Fundamentals of fluorescein angiography. Curr Concepts Ophthalmol. 2001;9(3):43-9.
8. Chopdar A. Manual of fundus fluorescein angiography. London, England: Butterworths;1989.
9. Kanski JJ. Acquired macular disorders. In: Kanski JJ. Clinical ophthalmology: a systemic approach. 4th ed. New York: Butterworth-Heinemann; 1999:396-436.
10. Garcia CR, Rivero ME, Bartsch DU, et al. Oral fluorescein angiography with the confocal scanning laser ophthalmoscope. Ophthalmology. 1999 Jun;106(6):1114-8.
11. Alexander LJ. Exudative and nonexudative macular disorders. In: Alexander LJ. Primary care of the posterior segment. 3rd ed. New York: McGraw-Hill; 2002:75-208.
12. López-Sáez MP, Ordoqui E, Tornero P, et al. Fluorescein-induced allergic reaction. Ann Allergy Asthma Immunol. 1998 Nov;81(5 Pt 1):428-30.
13. Gupta D. Fluorescein angiography refresher course. Rev Optom. 2001;138(11):60-6.
14. Kramer M, Mimouni K, Priel E, et al. Comparison of fluorescein angiography and indocyanine green angiography for imaging of choroidal neovascularization in hemorrhagic age-related macular degeneration. Am J Ophthalmol. 2000 Apr;129(4):495-500.
15. Yang CS, Lin CL, Lee FL, et al. Digital indocyanine green angiography in chorioretinal diseases. Chin Med J. 1995 Dec;56(6):411-7.
16. Reichel E, Duker JS, Puliafito CA. Indocyanine green angiography and choroidal neovascularization obscured by hemorrhage. Ophthalmology. 1995 Dec;102(12):1871-6.
17. Howe L, Stanford M, Graham E, Marshall J. Indocyanine green angiography in inflammatory eye disease. Eye (Lond). 1998;12(Pt 5):761-7.
18. Howe LJ, Woon H, Graham EM, et al. Choroidal hypoperfusion in acute posterior multifocal placoid pigment epitheliopathy. An indocyanine green angiography study. Ophthalmology. 1995 May;102(5):790-8.
19. Fernandes LH, Freund KB, Yannuzzi LA, et al. The nature of focal areas of hyperfluorescence or hot spots imaged with indocyanine green angiography. Retina. 2002 Oct;22(5):557-68.
20. Berger JW, Maguire MG, Fine SL. The macular photocoagulation study. In: Kertes PJ, Conway MD (eds.). Clinical trials in ophthalmology: a summary and practice guide. 1st ed. Baltimore: Williams & Wilkins; 1998:71-96.
21. Guyer DR, Yannuzzi LA, Slakter JS, et al. Digital indocyanine-green videoangiography of occult choroidal neovascularization. Ophthalmology. 1994 Oct;101(10):1727-35.
22. Guyer DR, Yannuzzi LA, Slakter JS, et al. Classification of choroidal neovascularization by digital indocyanine green videoangiography. Ophthalmology. 1996 Dec;103(12):2054-60.
23. Chang TS, Freund KB, de la Cruz Z, et al. Clinicopathologic correlation of choroidal neovascularization demonstrated by indocyanine green angiography in a patient with retention of good vision for almost four years. Retina. 1994;14(2):114-24.
24. Lauer AK, Wilson DJ, Klein ML. Clinicopathologic correlation of fluorescein and indocyanine green angiography in exudative age-related macular degeneration. Retina. 2000;20(5):492-9.
25. Atmaca LS, Batioglu F, Atmaca P. Evaluation of choroidal neovascularization in age-related macular degeneration with fluorescein and indocyanine green videoangiography. Ophthalmologica. 1996;210(3):148-51.
26. Schmitt JM. Optical coherence tomography: a review. IEEE J Sel Top Quantum Electron. 1999 July/Aug;5(4):1205-15.
27. Fercher AF, Drexler W, Hitzenberger CK, Lasser T. Optical coherence tomography - principles and applications. Rep Prog Phys. 2003 Feb;66(2):239-303.
28. Bressler N, Ahmed I. The Stratus OCT Primer: Esssential OCT. Jena, Germany: Carl Zeiss Meditec;2006:1-36.
29. Hassenstein A, Meyer CH. Clinical use and research applications of Heidelberg retinal angiography and spectral-domain optical coherence tomography - a review. Clin Experiment Ophthalmol. 2009 Jan;37(1):130-43.
30. Kiernan DF, Mieler WF, Hariprasad SM. Spectral-domain optical coherence tomography: a comparison of modern high-resolution retinal imaging systems. Am J Ophthalmol. 2010 Jan;149(1):18-31.
31. Kiernan DF, Hariprasad SM, Chin EK, et al. Prospective comparison of cirrus and stratus optical coherence tomography for quantifying retinal thickness. Am J Ophthalmol. 2009 Feb;147(2):267-75.
32. Otani T, Kishi S. Correlation between optical coherence tomography and fluorescein angiography findings in diabetic macular edema. Ophthalmology. 2007 Jan;14(1):104-07.
33. Kozak I, Morrison VL, Clark TM, et al. Discrepancy between fluorescein angiography and optical coherence tomography in the detection of macular disease. Retina. 2008 Apr;28(4):538-44.
34. Pai SA, Shetty R, Vijayan PB, et al. Clinical, anatomic, and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. Am J Ophthalmol. 2007 Apr;143(4):601-06.
35. Van de Moere A, Sandhu SS, Talks SJ. Correlation of optical coherence tomography and fundus fluorescein angiography following photodynamic therapy for choroidal neovascular
membranes. Br J Ophthalmol. 2006 Mar;90(3):304-6.
36. Kaiser PK, Blodi BA, Shapiro H, et al. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007 Oct;114(10):1868-75.
37. Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol. 1981 Oct;92(4):466-81.
38. Frangieh GT, Green WR, Baraquer-Somers E, Finkelstein D. Histopathologic study of nine branch retinal vein occlusions. Arch Ophthalmol. 1982 Jul;100(7):1132-40.
39. Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010 Sep;117(9):1792-9.
40. Imamura Y, Fujiwara F, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009 Nov-Dec;29(10):1469-73.
41. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009;54(1):1-32.
42. Maheshwary A, Oster S, Yuson R, et al. The association between percent disruption of the photoreceptor inner segment- outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010 Jan-Feb;150(1):63-67.
43. Ghazi NF, Ciralsky JB, Shah SM, et al. Optical coherence tomography findings in persistent diabetic macular edema: the vitreomacular interface. Am J Ophthalmol. 2007 Nov;144(5):747-54.
44. Sakamoto A, Nishijima K, Kita M, et al. Association between foveal photoreceptor status and visual acuity after resolution of diabetic macular edema by pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009 Oct;247(10):1325-30.
45. Do DV, Cho M, Nguyen QD, et al. Impact of optical coherence tomography on surgical decision making for epiretinal membrane and vitreomacular traction. Retina. 2007 June;27(5):552-6.
46. Diabetic Retinopathy Clinical Research Network, Browning DJ, Glassman AR, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007 Mar;114(3):525-36.
47. Lima VC, Yeung L, Castro LC, et al. Correlation between spectral domain optical coherence tomography findings and visual outcomes in central retinal vein occlusion. Clin Ophthalmol. 2011;5:299-305.
48. Krebs I, Hagen S, Brannath W, et al. Repeatability and reproducibility of retinal thickness measurements by optical coherence tomography in age-related macular degeneration. Ophthalmology. 2010 Aug;117(8):1577-84.
49. Coscas G, Coscas F, Zucchiatti I, et al. SD-OCT pattern of retinal venous occlusion with cystoid macular edema treated with Ozurdex. Eur J Ophthalmol. 2011 Sep-Oct;21(5):631-6.
50. Montero JA, Ruiz-Moreno JM. Optical coherence tomography characterization of idiopathic central serous chorioretinopathy. Br J Ophthalmol. 2005 May;89(5):562-4.
51. Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol. 1967 Mar;63(3):Suppl:1-139.
52. Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994;14(3):231-42.
53. Cukras C, Wang YD, Meyerle CB, et al. Optical coherence tomography-based decision making in exudative age-related macular degeneration. Eye (Lond). 2010 May;24(5):775-83.
54. Smiddy WE. Economic implications of current age-related macular degeneration treatments. Ophthalmology. 2009 Mar;116(3):481-7.
55. Park SS, Truong SN, Zawadzki RJ, et al. High-resolution Fourier-domain optical coherence tomography of choroidal neovascular membrane associated with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010 Aug;51(8):4200-6.
56. Khurana RN, Dupas B, Bressler NM. Agreement of time-domain and spectral-domain optical coherence tomography with fluorescein leakage from choroidal neovascularization. Ophthalmology. 1010 Jul;117(7):1376-80.
57. Brar M, Kozak I, Cheng L, et al. Correlation between spectral-domain optical coherence tomography and fundus autofluorescence at the margins of geographic atrophy. Am J Ophthalmol. 2009 Sep;148(3):439-44.

