In 2013, officials from the World Economic Forum contended that “the greatest risk to human health comes in the form of antibiotic-resistant bacteria.”1 Representatives from the US Centers for Disease Control and Prevention (CDC) largely agree with this grim assessment and suggest that inappropriate antibiotic use by both patients and physicians is a major contributing factor to escalating rates of microbial resistance.2
So, how can we best address this problem and limit the proliferation of resistant bacteria? For starters, clinicians must be more judicious when prescribing antibiotics for their patients. Additionally, we need to consider drug delivery models that best address any possible barriers to compliance––especially in patients who are most likely to be carriers of resistant bacteria.
The Roots of Resistance
One frequently cited explanation for increased rates of bactericidal resistance is unwarranted antibiotic prescribing for the management of cold- or flu-like symptoms. For example, CDC researchers have suggested that approximately 85% of all upper-respiratory infections are viral in nature, and that the use of antibiotics in these instances is superfluous and ineffective.2
According to a global survey of prescribing doctors published in 2007, the three main reasons for overprescribing antibiotics for viral infections included diagnostic uncertainty, time/pressure-related demands and patient request.3 Furthermore, the survey indicated that even when antibiotics are appropriately prescribed, patients aren’t always compliant with the recommended treatment regimen. Specifically, the data showed that roughly one out of five patients stopped taking their antibiotics earlier than prescribed because “they started feeling better.”3
The prophylactic use of topical antibiotics following ocular surgery or at injection sites also may precipitate resistance. In 2011, Stephen Kim, MD, and associates determined that repeated exposure to topical fluoroquinolones and azithromycin after intravitreal anti-VEGF injection was associated with increased multi-drug resistance in coagulase-negative Staphylococci (CNS).4 Additionally, his research team noted that more than 80% of CNS isolates were resistant to at least three antibiotics, and that more than 65% were resistant to at least five antibiotics.4
Basic Pharmacodynamics
To better understand how antibiotic agents eradicate bacteria and fight infection, it is important to distinguish between pharmacokinetics (PK) and pharmacodynamics (PD). Pharmacokinetic measurements are used to calculate the half-life of antimicrobial concentrations in the body, while pharmacodynamic measurements help researchers evaluate the relationship between those concentrations and their comprehensive antimicrobial effect.
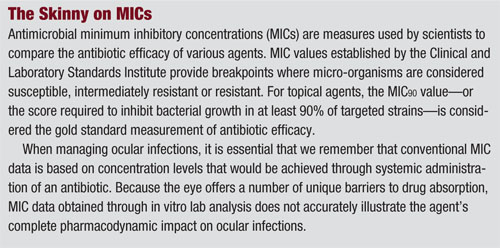
Traditionally, antibiotic dosing regimens have been determined by PK parameters only––such as rates of drug absorption, distribution, metabolism and elimination. However, PD measurements have become increasingly important within the last decade because they may be strategically employed to counteract or prevent resistance.
Three key PD metrics frequently used to enhance the therapeutic efficacy and bactericidal activity of antibiotic agents are:5
• T> MIC, or time above the minimum inhibitory concentration (MIC), is used to measure the duration a drug remains available at a concentration that’s greater than the MIC for a given pathogen. This parameter also is used to define the time-dependent killing of a pathogen.
• AUC:MIC ratio is the relationship between the area under the curve (i.e., the total exposure of an antibiotic to an organism) at 24 hours and the MIC.
• Cmax:MIC is the maximum detectable concentration of the drug in target tissue following dosing, with respect to the MIC.
The relative importance of each PD parameter to a drug’s antibacterial activity varies among agents.5 For the fluoroquinolones, researchers have estimated that a Cmax:MIC90 ratio of at least 10 and an AUC:MIC90 ratio of at least 100 are required to predict microbiological and clinical efficacy (see “Moxi’s MICs for MRSA,” page 44).6

Limitations of Empirical Treatment
In an ideal world, eye care professionals would have access to point-of-care diagnostic systems that would identify the causative organism within minutes of culturing. Then, such a system could offer guidance with regard to which antibiotic(s) would be the most appropriate. Until that day arrives, however, clinicians will be forced to rely on standard culture and sensitivity testing via local laboratories and hospitals.
Considering growth and replication rates for most bacterial species, standard culture and sensitivity testing usually takes three or four days to provide information. Given this delay, we’re often forced to treat suspected bacterial infections empirically.
While assumptions can be made about what pathogen(s) are involved based on the nature and location of the infection, a diagnosis made on clinical appearance alone is not always reliable. Further, without having the culture results in hand, the organism’s possible level of bactericidal resistance is largely indeterminable.
Therefore, whether faced with a sight-threatening infection or the need for prophylaxis around surgery, doctors most often turn to the latest generation topical fluoroquinolones––moxifloxacin, gatifloxacin and besifloxacin. These agents are a fairly safe bet, because they are very well tolerated (even at frequent dosing intervals) and offer an enhanced spectrum of activity against the most common ocular pathogens.
Nonetheless, without positive culture results, even frequent use of the most advanced fluoroquinolones can increase the likelihood of microbial resistance.
Challenges of Ocular Drug Delivery
The average tear film is approximately 7µL by volume. When a single eye drop (30µL to 50µL) is applied to the eye, a fair amount of drug is lost through epiphora and rapid tear clearance secondary to reflex blinking. These actions, in turn, contribute to decreased drug bioavailability.7
In addition, systemic absorption further promotes topical drug loss. Such inadvertent uptake may occur either directly through blood capillaries in the conjunctiva or indirectly via the nasolacrimal duct system.8
Because of the eye’s rapid tear clearance mechanism, patients who use topical agents with high drug concentrations and poor retention times intermittently experience low or even undetectable medication levels on the ocular surface. So, if the patient or caregiver fails to administer the drops according to the indicated dosing schedule, drug concentrations may fall below the levels necessary to kill the pathogen and avoid relapse.

Novel Delivery Systems
Former US Surgeon General C. Everett Koop is credited with a highly profound assertion: “Drugs don’t work in patients who don’t take them.”9 With specific regard to topical ophthalmic drugs, this statement couldn’t be more accurate. In my experience, it is much easier to ensure that patients swallow pills than instill drops in their eyes. Fortunately, advanced topical drug delivery systems may help our patients clear this hurdle. Here are a few of note:
• DuraSite. In an effort to combat poor compliance and low drug retention times, topical medications may be combined with a sustained drug delivery vehicle, such as DuraSite (InSite Vision). To date, two commercially available anti-infective agents have been formulated with the DuraSite vehicle––AzaSite (azithromycin, Merck) and Besivance (besifloxacin, Bausch + Lomb).
The mucoadhesive properties of the DuraSite vehicle facilitate enhanced drug residence times on the ocular surface. This, in turn, yields increased drug concentrations on the eye and improved clinical efficacy between dosing applications.10,11
• Sustained-release plugs. Ocular Therapeutix, Inc., currently is pioneering a novel punctal plug drug delivery system. The plug slowly delivers therapeutic levels of a drug to the ocular surface over a period of seven to 10 days as it dissolves. These plugs could be ideal for patients with tremendously poor compliance, such as those with severe arthritis or cognitive impairment.
In Phase I testing, researchers developed a single-arm, one-dose study in which 10 patients received a moxifloxacin-saturated punctal plug immediately following cataract surgery.12 Subjects were monitored over a 10-day period.
Primary outcome measures were plug retention and moxifloxacin levels above MIC levels required to halt the growth of various bacterial strains associated with conjunctivitis.
At the study’s conclusion, pharmacokinetic data indicated that tear sample drug levels were maintained between 2,000ng/ml and 3,000ng/ml over a seven-day duration in all 10 patients. These concentration levels are well above the MIC90 necessary to inhibit the bacterial growth of Staphylococcus aureus, Staphylococcus epidermidis and Streptococcus pneumoniae.12
• Contact lens delivery systems. Whether employing nanotechnology or differing biodegradable polymers such as PLGA acid, drug-eluting contact lenses can deliver therapeutic levels of antibiotic agents for up to one month.
Daniel S. Kohane, MD, and associates evaluated release medium from dual-polymer lenses that contained ciprofloxacin.13 The samples were collected after 28 days of use, and were tested against S. aureus isolates.
Ciprofloxacin showed complete killing of the S. aureus at an inoculum of 105 cells.13 It is important to note that, even at higher inoculum levels, the antibiotic still yielded complete bacterial inhibition––but rare isolates grew at low counts because of emerging bactericidal resistance.
The authors suggested that the drug release rate could be specifically customized by adjusting the molecular mass of the contact lens polymer as well as the volume of medication within the lens coating.13
Due to the limitations of standard culture and sensitivity testing, most ocular infections are treated empirically with broad-spectrum antibiotics.
However, the “shotgun” method of bacterial eradication––coupled with the rapid tear clearance of topical drops––can facilitate increased bactericidal resistance. Fortunately, novel drug delivery platforms that effectively incorporate pharmacodynamic research may successfully slow this trend.
Dr. Mangan is a fellow of the American Academy of Optometry and is board-certified in medical optometry. He’s also the founder of Beaumont Eye Consultants, LLC in Lexington, Ky. He has no direct financial interest in any of the products mentioned.
1. Howell L (ed.). World Economic Forum. Global Risks 2013: An Initiative of the Risk. Available at: www3.weforum.org/docs/WEF_GlobalRisks_Report_2013.pdf. Accessed March 24, 2014.
2. The US Centers for Disease Control and Prevention. About Antimicrobial Resistance: A Brief Overview. Available at: www.cdc.gov/drugresistance/about.html. Accessed March 27, 2014.
3. Pechère JC, Hughes D. Non-compliance with antibiotic therapy for acute community infections: a global survey. Int J Antimicrob Agents. 2007 Mar;29(3):245-53.
4. Kim SJ, Toma HS. Antimicrobial resistance and ophthalmic antibiotics: 1-year results of a longitudinal controlled study of patients undergoing intravitreal injections. Arch Ophthalmol. 2011 Sep;129(9):1180-8.
5. Ambrose PG, Bhavnani SM, Rubino CM, et al. Pharmacokinetics-pharmacodynamics
of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis. 2007 Jan 1;44(1):79-86.
6. Wright DH, Brown GH, Peterson ML, Rotschafer JC. Application of fluoroquinolone pharmacodynamics. J Antimicrob Chemother. 2000 Nov;46(5):669-83.
7. Zaki I, Fitzgerald P, Hardy JG, Wilson CG. A comparison of the effect of viscosity on the precorneal residence of solutions in rabbits and man. J Pharm Pharmacol. 1986 Jun;38(6):463-6.
8. Urtti A1, Rouhiainen H, Kaila T, Saano V. Controlled ocular timolol delivery: systemic absorption and intraocular pressure effects in humans. Pharm Res. 1994 Sep;11(9):1278-82.
9. Bonaccorso S, Sturchio JL. What information do patients need about medicines? Perspectives from the pharmaceutical industry. BMJ. 2003 Oct 11;327(7419):863-4.
10. Cambau E, Matrat S, Pan XS, et al. Target specificity of the new fluoroquinolone besifloxacin in Streptococcus pneumoniae, Staphylococcus aureus and Escherichia coli. J Antimicrob Chemother. 2009 Mar;63(3):443-50.
11. Ward KW, Lepage JF. Nonclinical pharmacodynamics, pharmacokinetics, and safety of BOL-303224-A, a novel fluoroquinolone antimicrobial agent for topical ophthalmic use. J Ocul Pharmacol Ther. 2007 Jun;23(3):243-56.
12. Chee SP. Moxifloxacin punctum plug for sustained drug delivery. J Ocul Pharmacol Ther. 2012 Aug;28(4):340-9.
13. Ciolino JB, Hoare TR, Iwata NG, et al. A drug-eluting contact lens. Invest Ophthalmol Vis Sci. 2009 Jul;50(7):3346-52.
14. Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008 Jun;145(6):951-8.
15. Haas W, Pillar C, Torres M, et al. ARMOR: A new prospective, multicenter surveillance study to determine the in vitro activity profile of besifloxacin and comparators against ocular pathogens from the US. Poster presented at: ARVO Annual Meeting; May 2-6, 2010; Fort Lauderdale, Fla. Poster D965.
16. Hesje CK, Sanfilippo CM, Haas W, et al. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolated from the eye. Curr Eye Res. 2011 Feb;36(2):94-102.

