Examining the optic nerve can be a daunting task. Its potential permutations are diverse, with individualized variations in color, size and even vascular supply.
A normal optic nerve head (ONH) usually is round or oval, mildly elevated and pink in color, with a centralized depression known as the cup. The horizontal diameter of a typical optic nerve is approximately 1.5mm.1
| |
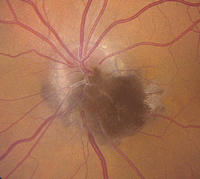
| 1. Melanocytoma with adjacent choroidal, retinal components and mild yellow exudation. |
When optic nerve abnormalities are detected, it is essential to differentiate between anatomical and pathological causes. This is because certain irregularities may require additional testing and/or intervention.
Optic nerve anomalies can be categorized as congenital or acquired. Congenital anomalies can be further subdivided into benign or pathologic. Acquired abnormalities are assumed to be pathologic, and generally are described with respect to optic nerve’s reaction to a given insult (i.e., cupping, swelling or atrophy).1
Benign Congenital Anomalies
• Melanocytomas are characteristically unilateral, very deeply
pigmented black or dark brown lesions that obscure all or part of the optic disc (figure 1).2 Their mean thickness is approximately 1.0mm; however, they may be noticeably elevated.2
Melanocytomas usually are located inferiorly on the optic nerve head, and up to 60% of cases involve either the retina or choroid.2 From a demographic standpoint, approximately two-thirds of all presentations are documented in whites, with just one-third seen in blacks.2 Although considered benign neoplasms, 1% to 2% of melanocytomas can transform into malignant melanoma.
• Tilted discs are characterized by an elevation of the superotemporal disc, posterior displacement of the inferonasal disc and situs inversus of the retinal vessels.1 They present bilaterally in 80% of patients.1
• Optic disc colobomas appear as sharply defined, white, bowl-shaped, inferiorly decentered excavations. Rarely is the entire disc affected. They can occur either unilaterally or bilaterally, with equal frequency.1
  |
|
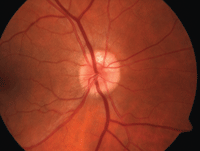
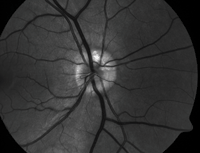
| 2, 3. Optic nerve head drusen located at approximately 12 o'clock, with a highlighted appearance on fundus autofluorescence (right). |
![]() Often, there can be associated colobomas of the iris and/or ciliary body. Further, patients with optic disc colobomas commonly experience significant refractive error and anisometropia.3
Often, there can be associated colobomas of the iris and/or ciliary body. Further, patients with optic disc colobomas commonly experience significant refractive error and anisometropia.3
It is important to note the potential association of optic disc colobomas with renal coloboma syndrome, which may cause devastating kidney problems. Because associated kidney diseases are potentially life threatening, individuals diagnosed with optic nerve colobomas should have a renal ultrasound.1,3
• Optic nerve head drusen are multilobed, globular concretions of calcium, amino and nucleic acids, mucopolysaccharides and sometimes iron.4 Ophthalmoloscopically, they appear as multiple, round to irregular, whitish-yellow dots within the surface of the nerve.1 Anomalous blood vessel patterns frequently are visible. The nerve itself is characteristically small and appears crowded. Such drusen are not usually visible at an early age, are said to be “buried” and appear to enlarge throughout life as they move closer to the surface and become more visible.
Optic nerve head drusen are often bilateral and have no
gender predilection. Adjunctive photographing techniques, such as fundus autofluorescence, may make the drusen appear more pronounced (figures 2 and 3). And, because optic nerve head drusen also may exhibit considerable elevation, they are best visualized with optical coherence tomography (figure 4).
 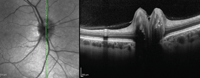
| |
| 4. Multiple focal, round, hyper-reflective masses with significant elevation. |
It is important to differentiate optic nerve head drusen from acquired bilateral disc edema or papilledema. Key clinical findings here include defined refractile aggregates, the presence of spontaneous venous pulsation and the absence of Paton’s lines.
Pathologic Congenital Anomalies
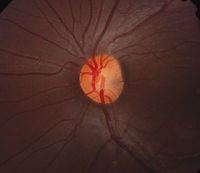
• Optic nerve pits are congenital abnormalities of the optic nerve due to incomplete closure of the fetal fissure. They are believed to occur during the first trimester of gestation, are usually less than one-half disc diameter in size and are more commonly located temporally.5 There are no known systemic or gender associations; however, a higher incidence of optic nerve pits is observed in those with basal encephalocele.5 Optic nerve pits are relatively rare, with an overall incidence of one in 11,000.
Circumpapillary chorioretinal atrophy with associated retinal pigment epithelium changes are commonly seen in those with optic nerve pits––especially if the pit is located near the disc margin.5 This can lead to visual field defects, particularly if the anomalies displace nerve fibers.
Optic pits are best seen clinically on dilated fundus evaluation and OCT imaging. They appear as grey, yellow or black excavations in the optic disc and are unilateral 95% of the time (figure 5).5 In 85% of cases, the optic nerve with the pit is larger than the fellow optic nerve.5
 |
|
| 5. Optic nerve pit. Note the subtle gray area located nasally to the optic nerve head's center. |
Optic nerve pits rarely affect visual acuity, unless the patient develops a serous macular detachment.5 The origin of the serous fluid is not completely understood, however.6 The most widely accepted theories are liquefied vitreous material gaining access to the subretinal space via the optic pit, or cerebrospinal fluid from the optic nerve leaking through the optic pit into the subretinal space.5 Histological studies involving dogs showed a connection between the vitreous and subretinal space; however, that finding has yet to be shown in human histopathological studies.
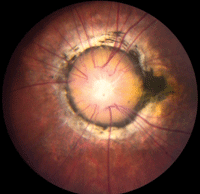
• Morning glory syndrome is a rare congenital dysplasia of the optic nerve.3 It almost always presents unilaterally and is more common in females.3 The condition was first described in 1970, and was named for its resemblance to the morning glory flower (figure 6).3 Upon physical examination, affected individuals frequently exhibit a large, excavated disc with glial tissue that is centrally surrounded by peripapillary pigment changes (which may include the macula).3 There is debate on the pathophysiology of this disc abnormality; however, many authorities now believe that it is a dysgenesis of mesodermal and ectoderm tissues.3
 | |
|
6. Morning glory disc. Note the dark pigment ring around the nerve, as well as centralized where the glial tissue remains.
|
Like optic nerve pits, morning glory syndrome may be associated with basal encephalocele––so, MRI often is indicated.
Unfortunately, visual acuity tends to range from 20/200 to
finger counting in patients
with morning glory syndrome; however, entering acuity can range anywhere from 20/20 to no light perception (NLP).3 While secondary vision loss is not progressive, 30% of morning glory patients develop serous retinal detachments secondary to small breaks in the peripapillary region.3
• Optic nerve hypoplasia is usually associated with an uncharacteristically small optic nerve head in which the number of axons is significantly reduced.3 It often occurs bilaterally (in 65% to 75% of cases), and is the third leading cause of blindness of children in the US (after cerebral damage during birth and retinopathy of prematurity).3
Optic nerve hypoplasia is characterized by a regression of axons due to apoptotic mechanisms catalyzed by the incomplete formation of other axons.3 Although causation can be spontaneous (especially in unilateral cases), some reports have indicated that premature birth, young maternal age, maternal type 1 diabetes or gestational diabetes, fetal infection, fetal alcohol syndrome, illicit drug abuse by the mother or the use of certain medications during pregnancy can precipitate optic nerve hypoplasia.3
Clinically, the most recognizable sign of optic nerve head hypoplasia is the “double ring sign,” in which a peripapillary ring sometimes surrounds the small nerve.3 Further, if the distance from the disc to macula is equal to or greater than 3DD, optic nerve hypoplasia is likely.3 Finally, most patients with optic nerve hypoplasia exhibit vessel tortuosity in the affected eye.3
Depending on the extent of axonal loss, visual acuity can be as good as 20/20 or as poor as NLP.3 Visual field defects are common, and clinicians often detect an afferent pupillary defect in patients with asymmetrical presentations.3 Visual evoked potential (VEP) testing can be helpful when the diagnosis is unclear.3 Patients with optic nerve hypoplasia would show a decreased amplitude in VEP, but a normal electroretinogram.3
• Leber’s hereditary optic neuropathy was first described in 1871 by Theodore Leber, when he documented a terrifying, non-Mendelian pattern of progressive vision loss in members of four families.7 Subsequent research indicated that the condition was caused by mutations to mitochondrial DNA.7 The prevalence of Leber’s hereditary optic neuropathy is as high as one in 25,000 in some populations, and it is by far the most common mitochondrial genetic disease.7 The condition commonly affects males in the second to forth decades of life.7
Leber’s presents in two phases: acute and chronic.7 In the acute phase, circumpapillary telangiectasia, nerve fiber layer swelling and vessel tortuosity are often noted; however, in 20% of cases, the optic nerve looks completely unremarkable.7
In the chronic phase, the nerve fiber layer degenerates, which causes optic atrophy.7 The acute phase typically persists for four to eight weeks after initial presentation. Then, after approximately six months, patients transition into the chronic phase.7
Patients with Leber’s hereditary optic neuropathy characteristically experience significant vision loss during the acute phase, frequently declining to 20/200 or worse before transitioning into the chronic disease phase.7
Acquired Pathologic Anomalies
• Ischemic optic neuropathy (ION) is the result of decreased blood flow to the optic nerve’s ganglion cells.8 ION may be either arteritic (AION) or non-arteritic (NAION).8,9 AION is caused by giant cell arteritis (GCA), while NAION may result from a variety of underlying vascular disorders.
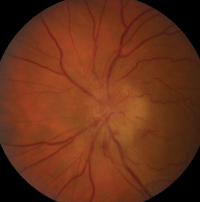
AION is characterized by a loss of blood flow to the most anterior portion of the optic nerve, which is supplied by the posterior ciliary artery.9 Visual acuity varies in patients with AION; however, all forms of ischemic optic neuropathy can potentially cause visual field defects, typically altitudinal (figure 7).8
 |
|
|
7. This patient presented with anterior ischemic optic neuropathy (AION). Note the hyperemic swelling of the optic nerve associated with many flame-shaped, peripapillary hemorrhages.
|
NAION is the most common form of ischemic optic neuropathy.9 Risk factors include hypertension, diabetes, atherosclerosis, sleep apnea, nocturnal hypotension, small discs and certain medications.9 NAION patients often present with painless, unilateral vision loss that has persisted for a few hours to several days.8
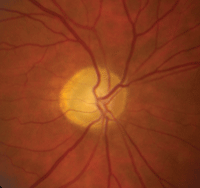
Upon examination, NAION patients show evidence of an afferent pupillary defect, sectoral disc edema and corresponding visual field loss.6 Over the course of a few weeks to months, the disc swelling subsides, resulting in sectoral nerve head pallor (figure 8).6
The clinical presentation of anterior arteritic ischemic optic neuropathy (AAION), is similar to that of AION; however, patients typically experience weight loss, headache, scalp tenderness, fever and malaise.6,8 These patients tend to be older and, if not treated within five to 10 days, will also develop vision loss in the contralateral eye up to 50% of the time.
The collateral branches of the long posterior and central arteries supply the posterior portion of the optic nerve. Thus, poor or compromised blood flow to this area results in a posterior ischemic optic neuropathy (PION).9 Like anterior ischemic optic neuropathy, PION can be arteritic or non-arteritic. Also, PION may be caused by significant blood loss, hypotension or general anesthesia during surgery.9 In these cases, resultant visual field loss tends to be both pronounced and bilateral, and can even result in complete blindness.9
 | |
|
8. Optic nerve appearance following AION, which features disc pallor located more temporally than nasally.
|
• Papilledema is defined as disc swelling secondary to increased intracranial pressure, and most often presents bilaterally.10 In the US, the most common cause of papilledema is idiopathic intracranial hypertension (also called pseudotumor cerebri).11 Most prevalent in obese women of childbearing age, chronic papilledema can lead to progressive optic atrophy.11 Although the exact etiology is unknown, overweight or obese individuals are at increased risk for papilledema.11
The clinical presentation includes bilateral disc swelling with obscuration of blood vessels, blurry disc margins, vessel tortuosity, hemorrhages and Paton’s lines. Patients often present with symptoms of blurred vision, transient vision loss, dizziness, tinnitus, headaches and visual field compromise.11 Visual prognosis tends to be quite good, with less than 25% of patients experiencing significant permanent vision loss.11
• Optic neuritis (ON) is inflammation of the optic nerve secondary to demyelinating diseases, such as multiple sclerosis (MS) and neuromyelitis optica.12 It tends to present in the second to fifth decades of life and has the highest incidence in white females.12 Although MS is the most common cause, ON also may be due to orbital or systemic infection (e.g., HIV or syphilis).5
Clinical signs and symptoms include acute unilateral vision loss that persists for hours or days before improving, pain upon eye movement, afferent pupillary defect and visual field defects.5,13 Because the optic nerve inflammation is retrobulbar in most cases, funduscopic examination often appears unremarkable. However, the optic nerve may start to show signs of pallor within four to six weeks after an acute event.5,6
Visual prognosis tends to be quite good after the first occurrence, as 75% of patients recover to 20/40 or better.6 That said, with each subsequent occurrence of ON, further visual decline is likely.
• Glaucoma is a progressive neuropathy of the optic nerve secondary to apoptosis, which eventually causes visual field defects. While the condition often is acquired, glaucoma also may be congenital or primary.6 Historically, glaucoma was thought to be a disease of increased eye pressure, and although increased intraocular pressure is a significant risk factor, it is no longer considered a defining characteristic.
Glaucomatous optic neuropathy differs from other diseases of the optic nerve, because it does not cause swelling or pallor.13 In glaucoma, localized areas of thinning (notches) most often occur in the inferior and superior quadrants of the optic nerve.5,6,14 Over time, we observe vertical enlargement of the cup-to-disc ratio, with corresponding ganglion cell loss.5,6 In some cases, disc hemorrhages precede ganglion cell loss.6 Initially, vision tends to be quite good; however, as the disease progresses, peripheral vision loss can eventually encompass the central field.6
Optic nerve disorders often have characteristic features, but in some cases may present similarly to other disorders. It is imperative to differentiate benign from pathologic conditions, as different cases may require extensive follow-up or pharmaceutical/surgical intervention. Regardless of the severity or associated risk, careful examination and documentation is necessary to properly identify and manage optic nerve anomalies. n
Dr. Laul is an instructor of ophthalmology at the Wilmer Eye Institute, Johns Hopkins School of Medicine, in Baltimore.
Dr. Fabrykowski is on staff at the Manhattan Eye, Ear and Throat Hospital Faculty Ophthalmology Practice, operated by Lenox Hill Hospital, in New York.
1. Golnik KC, Payesse EA, Torchia MM. Congenital Anomalies and Acquired Abnormalities of the Optic Nerve. Philadelphia: Wolters Kluwer; 2014.
2. Shields JA, Demirci H, Mashayekhi A, et al. Melanocytoma of the optic disc: a review. Surv Ophthalmol. 2006 Mar-Apr;51(2):93-104.
3. Dutton GN. Congenital disorders of the optic nerve: excavations and hypoplasia. Eye (Lond). 2004 Nov;18(11):1038-48.
4. Grippo TM, Rogers SW, Tsai JC, Lewis RA. Optic disc drusen: practical implications and management. Glaucoma Today. Available at: http://bmctoday.net/glaucomatoday/2012/02/article.asp?f=optic-disc-drusen. Accessed August 13, 2014.
5. Yanoff M, Duker J. Ophthalmology, 2nd ed. St. Louis: Mosby; 2004.
6. Kanski J. Clinical Ophthalmology: A Systematic Approach, 5th ed. London: Butterworth-Heinemann; 2003.
7. Man PY, Turnbull DM, Chinnery PF. Leber hereditary optic neuropathy. J Med Genet. 2002 Mar;39(3):162-9.
8. Athappilly G, Pelak VS, Mandava N, Bennett JL. Ischemic optic neuropathy. Neurol Res. 2008 Oct;30(8):794-800.
9. Hayreh S. Management of ischemic optic neuropathies. Indian J Ophthalmol. 2011 Mar-Apr;59(2):123-36.
10. Goosman M. Medscape. Papilledema. Available at: http://emedicine.medscape.com/article/1217204-overview. Accessed August 13, 2014.
11. Gans M. Medscape: Idiopathic intracranial hypertension. Available at: http:emedicine.medscape.com/article/1214410-overview. Accessed August 15, 2014.
12. Ergene E. Medscape: Adult optic neuritis. Available at: http://emedicine.medscape.com/article/1217083-overview. Accessed August 15, 2014.
13. Goodwin D. The differential diagnosis of an optic nerve disorder. Rev Optom. 2010 Apr;147(4):78-87.
14. Susanna R, Weinreb R. Answers in Glaucoma, 1st ed. Rio de Janerio, Brazil: Cultura Medica; 2005.

