A recent report indicates that 2014 was a good year for the contact lens field, with healthy growth.1 Currently, the contact lens market is estimated at approximately $2.5 billion in the United States and $7.6 billion worldwide. Despite this buoyant market, contact lens-related discomfort (CLD) remains one of the major problems associated with contact lens wear, with up to 50% of patients reporting CLD.2
An international panel of experts organized by the Tear Film and Ocular Surface Society (TFOS) defined CLD as: “a condition characterized by episodic or persistent adverse ocular sensations related to lens wear, either with or without visual disturbance, resulting from reduced compatibility between the contact lens and the ocular environment, which can lead to decreased wearing time and discontinuation of contact lens wear.” The majority of dissatisfied lens wearers report that this discomfort and dryness, particularly at the end of the day, are major reasons for cessation of contact lens wear; this discontinuation is believed to have huge financial ramifications for both the contact lens industry and clinical practice.3
The purpose of this article is to provide a brief overview of our current understanding of the impact of contact lens materials on CLD. In addition, this article covers recent developments and potential new applications of contact lenses.
How Lens Materials Impact CLD
The TFOS report on CLD showed that the onset of this condition is influenced by many factors, including some related to the patient and lens composition.4
There are several material-related factors believed to play a role in determining comfort during contact lens wear.5
These include water content and ionicity, oxygen transmissibility, modulus, dehydration, wettability, deposition and coefficient of friction.5
Interestingly, the TFOS report demonstrated that coefficient of friction was the only material-related factor that actually correlated with CLD. Other soft contact lens physical properties that were found to relate to CLD include denatured protein on contact lenses, lens replacement frequency, lens design, thickness/bulk and edge configuration.5
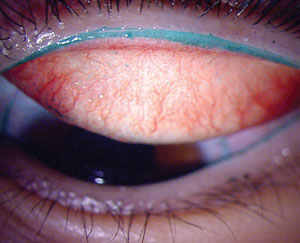
It is a well-established fact that CLD is multifactorial, and it is likely that an interaction exists between the posterior surface of the lens with the corneal surface, and also the anterior surface of the contact lens with the posterior surface of the eyelid during the blink. Within only a few minutes of wear, contact lenses rapidly attract various components from the tear film, including proteins, lipids and mucins.6 These deposits have the potential to alter the properties of the contact lens surface, which can impact the frictional forces created when the eyelid rubs against the contact lens during blinking. Moreover, the lenses can dehydrate, leading to an increase in lid/lens interaction due to a reduction in lens front-surface wettability and the development of epithelial staining due to pervaporation and subsequent desiccation.7 Lens friction may also be associated with clinically observable conditions, including lid wiper epitheliopathy (figure 1) and lid parallel conjunctival folds.8,9 Under extreme conditions, the mechanical interaction of the palpebral conjunctiva with the lens surface can lead to contact lens associated papillary conjunctivitis.10
Sufficient evidence suggests that different care solutions interact differently with contact lens materials and that this interaction is strongly dependent on the contact lens material properties and the composition of the lens care solution. Thus, clinicians should remember that the mechanisms contributing to symptomatology are significantly affected by the manner in which the components in a lens care product interact with the lens material.
Overall, further research studies are required to develop novel technologies that will reduce or eradicate the problems experienced by contact lens wearers.
Delivering the Goods
While contact lenses are primarily used as a means to correct vision, research in recent years has turned toward the use of lenses for other applications, including disease detection, drug delivery and myopia control. For instance, researchers have incorporated novel components into lens materials; these embedded contact lenses have been successfully used to monitor intraocular pressure and tear glucose levels.
|
|
|
|
|
Fig. 1. Lens friction may be associated with clinically observable conditions such as lid wiper epitheliopathy (above). Photo: Centre for Contact Lens Research |
• CLs for IOP monitoring. Glaucoma is a chronic, neurodegenerative disease considered to be one of the leading causes of irreversible blindness worldwide. While sound prediction methods for developing the disease remain uncertain, there has been a recent increased interest in developing methods to monitor IOP over a 24-hour period, as some studies have suggested fluctuations in IOP may be an important contributor to the risk of glaucoma progression. Technology capable of constant monitoring of IOP could be attractive to practicing clinicians who prefer to manage glaucoma efficiently.
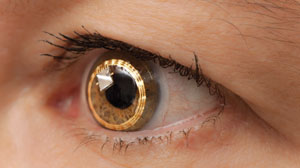
The Sensimed Triggerfish device (figure 2) is a contact lens sensor that has been successfully used to monitor intraocular pressure over a period of 24 hours.11,12 This sensor consists of two platinum/titanium sensing-resistive strain gauges capable of recording circumferential changes at the corneoscleral area.13 Together with a microprocessor, this sensor is embedded within a silicone-based disposable contact lens material. The disposable lens is available in three different base curves with an approximate thickness of 585µm at the centre and 260µm at the periphery.13 Triggerfish is currently available in several countries in Africa, Asia, Europe and South America. In North America, it is available in Canada and Mexico, but not approved for use in the United States.
• CLs for glucose monitoring. Diabetes is a global health crisis, and it’s only getting worse. In 2013, an estimated 382 million people had diabetes worldwide.14 The World Health Organization reported diabetes caused over 1.1 million deaths in 2005, and that death related to diabetes is estimated to double by 2020. Currently, diabetes is managed by monitoring blood glucose levels, an invasive procedure that patients with diabetes self-administer several times a day. Other bodily fluids such as urine, saliva and tears can also be monitored; indeed, it has been shown that tear glucose levels can be elevated in hyperglycemic individuals.15,16 But, while tears are easily accessible, collection of an adequate sample for testing is time consuming.
One means of determining tear glucose under investigation is to incorporate sensors within a contact lens to monitor tear glucose levels. Contact lens prototypes have been developed previously to monitor tear glucose levels; however, they were never made available for clinical trials.17-19 More recently, Google announced that its life sciences division, Google X, has been working on a contact lens that could monitor tear glucose levels. The lens would contain an embedded glucose sensor, wireless chip and antenna (figure 3). A tiny hole in the lens would enable the tear fluid to penetrate into the lens, where it would come in contact with the sensor. The antenna then transmits information to an external wireless device.
Because this lens is still in the design stage, the performance of this device in adverse environments and eyes of varying tear profiles is currently unknown; however, this could be a significant advancement that will likely lead to more medical and vision devices for future generations. Google’s smart contact lens is currently licensed to Novartis’ eye care division, Alcon Vision Care.
• Drug-eluting CLs. For years, contact lenses have been used as bandage lenses to manage pain and promote re-epithelialization following surgery or ocular trauma.20 In most of these cases, antibiotics and anti-inflammatory drugs are administered topically over the bandage lenses.19 Research has been ongoing, however, in a contact lens that is specifically developed for use as a drug-delivering therapeutic lens.20
|
|
| Fig. 2. The Triggerfish contact lens sensor monitors IOP over a 24-hour period. Photo: ©Sensimed AG |
|
The use of soft contact lenses as drug delivery devices was first proposed by Waltman and Kaufman in 1970 and, since then, more than 200 papers have been published that investigate the release of anti-glaucoma drugs, antibiotics, antiviral agents, epithelial growth factors and anti-inflammatory medications.
One advantage of using lenses as ocular drug delivery devices is their ability to overcome several barriers that limit the effective use of eye drops. When released by the lens, the drug is eluted directly into the post-lens tear film, where it would have prolonged contact time with the cornea, resulting in increased bioavailability.21 Such a method has been shown to be 35 times more efficient than conventional eye drops.22
Another advantage of using contact lenses as drug delivery devices is the ability to release the drug over an extended period of time. Extensive research has been conducted in the last few years to develop contact lenses that release drugs at a slow and sustained rate, and in vitro results obtained thus far have been extremely promising. Strategies such as molecular imprinting, vitamin E coatings and nanoparticles have been shown to be effective in retaining the drug within the contact lens.23-25 This is particularly useful in cases of ocular infections, such as microbial keratitis, where eye drop application should be hourly or even more frequent.26
Although there has been a significant progress in the last few years in the development of contact lenses capable of releasing drugs at a slow and sustained rate, one major limitation is a shortage of in vivo clinical studies that can validate the viability of the drug-delivering devices. Further, drug delivery is also limited by the molecular weight of the substance eluted. Overall, however, while the cost of such a system will likely be higher than the conventional eye drop method, the treatment efficacy and the system’s ability to reduce the frequency of topical application will likely make this commercially attractive to clinicians.
Controlling Myopia
Myopia affects an estimated 1.5 billion people worldwide (roughly 22% of the current global population).27
Approximately 33% of Americans are myopic, with roughly 2% of kindergarteners and 15% of high school students affected.27
Various strategies and treatment options to prevent myopia progression exist, including spectacles, contact lenses and anti-muscarinic medications.28
The prescription of contact lenses for myopia control in particular has recently increased.1
|
|

|
|
|
Fig. 3. This investigational "smart" contact lens monitors tear glucose levels with an embedded sensor. Photo: ©Google |
Rigid gas permeable contact lenses were found to have no evidence of effect on myopic children’s eye growth; however, progressive addition lenses (PALs) and bifocal spectacles were found to yield a small slowing of myopia progression.28 Furthermore, children wearing multifocal lenses, either PALs or bifocals, progressed on average 0.16D less than children wearing single-vision lenses.28 A more recent study that involved the use of contact lenses to reduce hyperopic defocus in the peripheral retina found that these contact lenses slow the progression of myopia by 40% per year.29 Thus, contact lenses designed to induce peripheral hyperopia appear to be an attractive, efficient and safe means of controlling the progression of myopia.
Several independent studies have shown orthokeratology (ortho-k) lenses slow ocular elongation.30,31 The changes induced with ortho-K treatment are directly related to the initial myopia level, and thus variable. These lenses are considered by many to be the most trustworthy index of myopia control; however, adverse events should be carefully evaluated. There have been case reports on severe microbial keratitis infections in children wearing these lenses.32
A few other contact lenses are also available for clinicians to try as a treatment option to prevent the progression of myopia, including the five-zone dual power MySight design from New Zealand and another lens based on the Fresnel design from Hong Kong.28
In conclusion, contact lens-related discomfort continues to be a major problem that can drastically impact the success of contact lens wear. Researchers, clinicians and the contact lens industry have made considerable efforts to identify the causes and manage this condition. Contact lens researchers have made significant strides in identifying the causes of this condition and are continuing to improve the properties of lens materials. Furthermore, several novel designs of lens materials that can be applied for conditions other than refractive correction have also been developed and launched. The development and innovations that have occurred over the past few years offer the promise of a wider application of contact lenses that could be attractive to both practitioners and patients.
Dr. Srinivasan is a research assistant professor and a clinical research manager at the Centre for Contact Lens Research (CCLR), School of Optometry and Vision Science, University of Waterloo, Canada. She is actively involved in various clinical trials conducted at the CCLR.
Dr. Subbaraman is the head of biological sciences and a senior clinical scientist at CCLR. He has authored several peer-reviewed and professional articles in the area of contact lens discomfort and tear film biochemistry, and has presented at national and international conferences.
Disclosure: Over the past three years, CCLR has received research support or honoraria from the following companies: Advanced Vision Research, Alcon, AlgiPharma, Allergan, Ciba Vision, CooperVision, Contamac US, Eleven Biotherapeutics, Essilor, Johnson & Johnson Vision Care, Ocular Dynamics, Oculus, Ocusense, TearScience, Visioneering Technologies. However, Drs. Srinivasan and Subbaraman do not serve on an advisory board or own shares in any optometric company.
1. Nichols J. Contact Lenses 2014. Contact Lens Spectrum 2015; January 2015.
2. Dumbleton K, Caffery B, Dogru M, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on epidemiology. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS20-36.
3. Fonn D. Targeting contact lens induced dryness and discomfort: what properties will make lenses more comfortable. Optom Vis Sci. 2007 Apr;84(4):279-85.
4. Nichols JJ, Willcox MD, Bron AJ, et al. The TFOS International Workshop on Contact Lens Discomfort: executive summary. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS7-TFOS13.
5. Jones L, Brennan NA, Gonzalez-Meijome J, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens materials, design, and care subcommittee. Invest Ophthalmol Vis Sci. 2013;54(11):TFOS37-70.
6. Bontempo AR, Rapp J. Protein and lipid deposition onto hydrophilic contact lenses in vivo. CLAO J. 2001;27(2):75-80.
7. Pritchard N, Fonn D. Dehydration, lens movement and dryness ratings of hydrogel contact lenses. Ophthal Physiol Opt. 1995;15(4):281-6.
8. Berry M, Pult H, Purslow C, Murphy PJ. Mucins and ocular signs in symptomatic and asymptomatic contact lens wear. Optom Vis Sci. 2008;85(1):E930-8.
9. Pult H, Purslow C, Berry M, Murphy PJ. Clinical tests for successful contact lens wear: relationship and predictive potential. Optom Vis Sci 2008;85(1):E924-9.
10. Donshik PC. Contact lens chemistry and giant papillary conjunctivitis. Eye Contact Lens. 2003;29(1 Suppl):S37-9; discussion S57-9, S192-4.
11. Mansouri K, Medeiros FA, Weinreb RN. Twenty-four-hour intraocular pressure patterns in a symptomatic patient after ab interno trabeculotomy surgery. Clin Ophthalmol 2014 7;8:2195-7.
12. Mansouri K, Medeiros FA, Tafreshi A, Weinreb RN. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucoma. Arch Ophthalmol 2012;130(12):1534-9.
13. Mansouri K, Weinreb R. Continuous 24-hour intraocular pressure monitoring for glaucoma—time for a paradigm change. Swiss Med Wkly 2012;142:w13545.
14. Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014 Feb;103(2):137-49.
15. Michail D, Vancea P, Zolog N. Sur l’elimination lacrymale du glucose chez les diabetiques. C R Soc Biol, Paris 1937;125:1095.
16. Michail D, Zolog N. Sur l’elimination lacrymale du glucose au cours dr l’hyperglycemie alimentaire. C R Soc Biol Paris 1937;126:1042.
17. Alexeev VL, Das S, Finegold DN, Asher SA. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin Chem 2004;50(12):2353-60.
18. Badugu R, Lakowicz JR, Geddes CD. Ophthalmic glucose sensing: a novel monosaccharide sensing disposable and colorless contact lens. Analyst 2004;129(6):516-21.
19. Badugu R, Lakowicz JR, Geddes CD. Noninvasive continuous monitoring of physiological glucose using a monosaccharide-sensing contact lens. Anal Chem 2004;76(3):610-8.
20. Karlgard CC, Jones LW, Moresoli C. Survey of bandage lens use in North America, October-December 2002. Eye Contact Lens 2004;30(1):25-30.
21. Hehl EM, Beck R, Luthard K, et al. Improved penetration of aminoglycosides and fluorozuinolones into the aqueous humour of patients by means of Acuvue contact lenses. Eur J Clin Pharmacol 1999;55(4):317-23.
22. Li CC, Chauhan A. Modeling ophthalmic drug delivery by soaked contact lenses. Ind Eng Chem Res 2006;45(10):3718-34.
23. Peng CC, Kim J, Chauhan A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing vitamin E diffusion barriers. Biomaterials 2010;31(14):4032-47.
24. Phan CM, Subbaraman L, Liu S, Gu F, Jones L. In vitro uptake and release of natamycin Dex-b-PLA nanoparticles from model contact lens materials. J Biomater Sci Polym Ed 2014;25(1):18-31.
25. Ciolino JB, Hudson SP, Mobbs AN, et al. A prototype antifungal contact lens. Invest Ophthalmol Vis Sci 2011;52(9):6286-91.
26. Yolton DP. Antiinfective drugs. In: Bartlett JD, Jaanus SD, ed. Clinical Ocular Pharmacology. 4th edition ed. Woburn, MA: Butterworth-Heineman, 2001: 219-64.
27. Yu L, Li ZK, Gao JR, Liu JR, Xu CT. Epidemiology, genetics and treatments for myopia. Int J Ophthalmol. 2011;4(6):658-69.
28. Walline JJ, Lindsley K, Vedula SS, et al. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev 2011 Dec 7;(12):CD004916.
29. Holden B, Sankaridurg P, Smith E, et al. Myopia, an underrated global challenge to vision: where the current data takes us on myopia control. Eye (Lond) 2014;28(2):142-6..
30. Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutierrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Invest Ophthalmol Vis Sci 2012;53(8):5060-5.
31. Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci 2012;53(11):7077-85.
32. Tran T, Samarawickrama C, Petsoglou C, Watson S. Recent cluster of childhood microbial keratitis due to orthokeratology. Clin Experiment Ophthalmol 2014;42(8):793-4.

