  |
A 55-year-old black male with a history of Type 2 diabetes and hypertension presented urgently for evaluation. A regular patient to the clinic, he had been seen six months ago for a comprehensive examination and diagnosed with moderate nonproliferative diabetic retinopathy (DR)in both eyes. At today’s visit, he reported a two-day history of vision loss, which developed abruptly. He said he experienced unusual flashes of light, followed by a sudden “darkening” affecting his left eye.
Evaluation
Examination revealed corrected acuity of 20/25 OU. Pupils were normally reactive and ocular motilities were unrestricted. Visual field testing by confrontation demonstrated severe depression of the superior temporal quadrant of the left eye, but was also noted to affect the superior nasal quadrant of the right eye. Biomicroscopy revealed healthy anterior segment structures; dilated funduscopy showed moderate nonproliferative DR, but no evidence of neovascular scaffolding, vitreous hemorrhage or retinal detachment in either eye. Ultimately, an automated field analysis was performed, demonstrating a dense, homonymous hemianopic defect of the left visual field, denser above than below the horizontal midline.
Although the patient’s chief complaint seemed to indicate a retinal etiology, testing quickly confirmed this was a neuro-ophthalmic event.
Wise clinicians analyze patients’ complaints within the context of the ocular and medical history to arrive at a logical presumptive diagnosis. Sometimes, however, the most obvious diagnosis is not the correct one. We learned this principle from professor Lou Catania, OD, quoting Hickam’s dictum: “The patient may have as many diseases as he or she pleases.”
In this case, DR complications or rhegmatogenous retinal detachment appeared likely, but a thorough evaluation revealed another suspect: cerebrovascular disease.
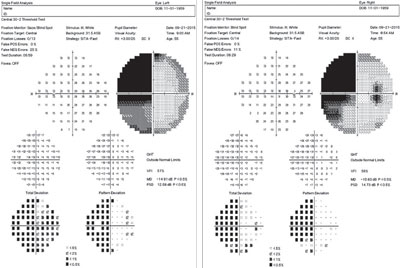 | |
| This 55-year-old diabetic patient’s field analysis shows a dense, homonymous hemianopic defect of the left visual field, denser above than below the horizontal midline. |
A Roadmap to the Brain
Visual field testing provides insight into the integrity of the visual pathway. The visual pathway is exquisitely organized as it traverses the entire length of the brain, from an area just beneath the frontal lobe to the tip of the occipital cortex. Nerve fibers originating in the nasal retina cross at the level of the optic chiasm, traveling with contralateral temporal fibers to the lateral geniculate nucleus where they synapse. Post-geniculate fibers split into superior and inferior pathways, traveling through the parietal and temporal lobes, respectively, as they course posteriorly in the brain. As they reach the occipital lobe (the primary visual cortex), the superior and inferior fibers are reunited.
Because of this extraordinarily detailed and consistent arrangement, lesions of the visual pathway result in predictable and repeatable visual field defects. The late Larry Gray, OD, used to tell us “visual fields read like a roadmap to the brain.” The implication is, if you can understand the architecture of the pathways and the nuances of visual field defects, you can potentially pinpoint the precise location of the intracranial pathology.
As we know, purely unilateral field defects typically reflect pre-chiasmal lesions, either within the retina or optic nerve. Lesions of the chiasm—most commonly pituitary gland tumors or craniopharyngiomas—result in bitemporal, heteronymous field defects. Post-chiasmal lesions result in homonymous field defects, i.e., affecting the same side (right or left) of the visual field and respecting the vertical midline. Damage to the optic radiations in the temporal or parietal lobe results in a quadrantanopia, impacting the superior or inferior field, respectively. These field defects will cross neither the vertical midline nor the horizontal midline. Lesions of the occipital lobe result in homonymous hemianopias, contralateral to the side of the lesion (i.e., a right-sided field defect with a left-sided lesion and vice-versa).
Also important in the localization of visual field defects is the concept of congruity. Congruity refers to the similarity in shape and depth of the visual field defect in each eye. In essence, the more the field loss in one eye appears to be a “carbon copy” of the other, the greater the level of congruity.
For pathology affecting the post-chiasmal pathways, the accepted convention is that greater congruity is associated with more posteriorly located lesions.1 However, this is not an absolute rule. One study demonstrates congruous visual field defects encountered in 350% of lesions to the optic tract or optic radiations, although 84% of occipital lobe lesions produced congruous field defects.2 Also, the vast majority of incongruous visual field defects occur with lesions to the optic radiations, which are located more anteriorly.2 One important caveat to this rule is that only incomplete homonymous hemianopias may be described as congruous or incongruous. By definition, an incomplete hemianopia spares at least part of the vision on the affected side. Complete homonymous hemianopias have no localizing value, except to say that they are post-chiasmal.1
Findings
Our patient was found to have an incomplete, moderately congruous, left homonymous hemianopia, denser above than below the horizontal midline. Due to the abrupt onset of symptoms, we suspected a cerebrovascular accident affecting the posterior optic radiations or anterior occipital cortex. The pattern of the visual field defect is also more indicative of stroke than other intracranial abnormalities, such as traumatic brain injury, cerebral hemorrhage or compressive tumor.2 The patient was advised that he had likely suffered an isolated stroke which caused him to experience vision loss. He later revealed he’d been suffering from a headache on the right side, behind his ear (the occipital region).
Further Recommendations
The patient was referred to his primary care physician with a request for an MRI with contrast of the area in question, to be performed within 24 to 48 hours. Additionally, a comprehensive neurological evaluation was requested to rule out any additional compromise. Once confirmed, treatment goals for isolated stroke are aimed at controlling modifiable risk factors that could predispose to subsequent CVAs. This includes tighter control of comorbidities such as diabetes, hypertension, hypercholesterolemia, hyperlipidemia and obesity. The use of aspirin as a platelet anti-aggregant may be advisable in those with significant intracranial arterial stenosis.3 Smoking cessation (including avoidance of second-hand smoke), a low-fat diet and regular exercise are also typically encouraged.4
Optometric management depends on the extent of field loss and the degree of recovery following the stroke. According to research, the majority of recovery occurs within the first 60 days after the initial event, and recovery after six months is highly unlikely.5 Patients with residual field deficit following stroke may benefit from vision rehabilitation, including such elements as prismatic correction and compensatory training to improve visual search abilities.1 Vision restorative training (VRT) may provide a mechanism to regain visual function at the border of the visual field defect, although studies with this system have yielded conflicting and inconclusive results.6,7
Our thanks to the following individuals for helping to inspire this article: Mr. Matthew Hennen (SCO ’16), Dr. Leah Stevens and Dr. Robert Goodwin (via ODs on Facebook).
1. Goodwin D. Homonymous hemianopia: challenges and solutions. Clin Ophthalmol. 2014 Sep 22;8:1919-27.
2. Kedar S, Zhang X, Lynn MJ, et al. Congruency in homonymous hemianopia. Am J Ophthalmol. 2007 May;143(5):772-80.
3. Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005 Mar 31.;352(13):1305-16.
4. Meschia JF, Bushnell C, Boden-Albala B, et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Dec;45(12):3754-832.
5. Ali M, Hazelton C, Lyden P, et al; VISTA Collaboration. Recovery from poststroke visual impairment: evidence from a clinical trials resource. Neurorehabil Neural Repair. 2013 Feb;27(2):133-41.
6. Kasten E, Bunzenthal U, Sabel BA. Visual field recovery after vision restoration therapy (VRT) is independent of eye movements: an eye tracker study. Behav Brain Res. 2006 Nov 25;175(1):18-26.
7. Pelak VS, Dubin M, Whitney E. Homonymous hemianopia: a critical analysis of optical devices, compensatory training, and NovaVision. Curr Treat Options Neurol. 2007 Jan;9(1):41-7.

