Optometrists’ primary responsibility in cataract surgery is to ensure good medical eye care during the postoperative period. Fortunately, the advent of small-incision phacoemulsification techniques now allows for earlier recovery of visual function.1,2
| |
|
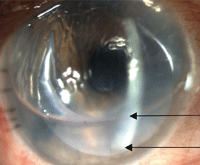
Securely attached DSEK graft and appropriate air bubble following reversal of pupillary block.
|
At the same time, with increased emphasis on reducing dependency on corrective eyewear following cataract surgery, our care is evolving. Patients are now regarding cataract surgery as more of a refractive procedure, and expect us to help them achieve an excellent visual outcome.
Thus, the care we now provide extends from a solid understanding of medical complications to a vigilant attentiveness to the vision expectations of the patient.3 As a result, comanaging providers must remain attuned to the nuances of specialty intraocular lenses, and return patients for surgical intervention in a time-appropriate manner.
This article outlines three interesting cases that highlight both important roles as postsurgical providers.
Case #1: Pupillary Block After Combined DSEK
• History. A 79-year-old white female presented for a one-day follow-up after combined Descemet’s stripping endothelial keratoplasty (DSEK) and cataract extraction with monofocal IOL implantation in the right eye. She reported no abnormal complaints of ocular discomfort, but had noticed a persistent headache since the procedure.
Topical postoperative medications included ofloxacin 0.3% QID, prednisolone acetate 1% QID and bromfenac 0.09% QD. Over-the-counter lubricants and Muro 128 solution (Bausch + Lomb) were also prescribed for the early postoperative period. The patient had a history of endothelial dystrophy and had already undergone DSEK in the fellow eye five months prior.
• Diagnostic data. The patient’s visual acuity on day one was limited to hand motion OD. The endothelial graft was well centered and the cornea showed 1 to 2+ stromal edema, as expected. There was no graft dehiscence.
Anterior bowing of the iris was visible 360° with an occluded angle, despite a patent prophylactic peripheral iridectomy inferiorly. The anterior chamber was maximally filled with air in place of aqueous humor. Intraocular pressure by applanation tonometry measured 46mm Hg OD.
• Diagnosis and management. This patient had pupillary block. After alerting the surgeon, we decided to immediately lower the intraocular pressure behind the biomicroscope.
With a metal dilator, we applied pressure just posterior to the sutured temporal incision. This depression allowed immediate escape of air to achieve a more defined air bubble within the anterior chamber. As a result, the anterior chamber structures returned to their normal state and the patient’s IOP reduced to 6mm Hg. Her symptoms abated just as quickly.
• Discussion. Air-bubble management following endothelial graft surgery is an important responsibility of the postoperative care provider. Pupillary block is a serious post-op complication and could result in permanent vision loss if improperly managed.
To review, the perioperative injection of air into the anterior chamber during surgery is intended to create tension against the graft and prevent dehiscence. Despite an uncomplicated surgical case, air can inadvertently become trapped behind the iris, which can lead to irido-corneal touch. (Prophylactic peripheral iridectomies are routinely created during endothelial graft cases to mitigate undesired pupillary block. The air bubble is expected to assume about 60% of the anterior chamber’s height on the first day after surgery, and to not encompass the iridectomy.) This is why patients are discouraged from bending forward in the immediate postoperative period. In addition, the patient is asked to spend most of the first 24 hours on his or her back, as the tension of air against the graft is most effective when the patient is in a supine position.
Because the bubble interferes with visual acuity, patients are reassured of its reabsorption over the following few days.
Take great care when applanating corneas with new endothelial grafts due to graft instability. Prophylactic oral acetazolamide or IV mannitol may be administered perioperatively to deter postoperative pressure rise and to potentially minimize the need for applanation tonometry on day one. If needed, pneumotonometry is the preferred method of assessing intraocular pressure this early in the postoperative period.
Case #2: Toric IOL Rotation
• History. A 53-year-old white male returned to the office for a one-week follow-up after uncomplicated cataract extraction with Trulign Toric IOL (Bausch + Lomb) insertion in the right eye. He noted improved vision in this eye when compared with his vision before the cataract procedure, but did not believe that the vision was as sharp as that achieved postoperatively in his left eye. (This fellow eye had also been implanted with a Trulign Toric IOL one month prior.)
• Diagnostic data. Uncorrected vision in the right eye measured 20/60 at distance, 20/32 at intermediate and 20/32 at near. The eye best corrected to 20/20 after the manifest refraction yielded +0.25-2.00x115.
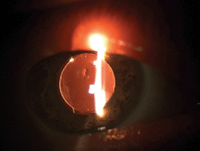
Slit lamp biomicroscopy confirmed a well-healing eye with expected anterior chamber reaction and a clearing corneal stroma. Both clear corneal incisions were sound and had not required suturing. Posterior pole evaluation revealed a healthy, unremarkable posterior segment. The IOL was in its intended posterior vault formation—but the axis of rotation was at 109°. This contrasted with the surgeon’s intended axis of 89°.
• Diagnosis and management. This patient had undesired postoperative astigmatism due to IOL rotation, and returned to the cataract surgeon in a timely fashion. At this consultation, the cataract surgeon ordered a surgical reposition of the IOL, which he performed the following day using ORA wavefront aberrometry (WaveTec Vision) to precisely realign the IOL.
One week after the repositioning procedure in the right eye, his distance, intermediate and near acuities were 20/15-, 20/20 and 20/40 respectively with a plano refraction.
• Discussion. Postoperative examination of specialty intraocular lenses requires additional attention to detail. Both the Trulign Toric IOL and the Crystalens accommodating IOL (Bausch + Lomb) are designed to be optimally positioned for translation when the haptic hinges are in a posteriorly flexed state within the posterior capsule. At the slit lamp, there should be visible space between the IOL optic and the posterior iris plane. The IOL is said to be in a state of posterior vault.
But, Z-formation (also called Z-syndrome) can occur when one of the two hinges moves anteriorly, creating a tilted IOL optic that typically induces undesired astigmatism. In addition, both hinges may flex forward anteriorly, creating a negative vault. This would likely lead to the patient becoming more myopic.
The rotational stability of a toric IOL determines the effectiveness of the outcome; there is a 3.3% loss of effectiveness for each degree of rotation.4 Therefore, assuming the degree of rotation is the same, a higher-powered toric IOL off alignment will yield more undesired astigmatism than a misaligned low-powered toric IOL. Toric designs, such as the AcrySof IQ Toric IOL (Alcon), have shown excellent rotational stability and rotate less than 4° on average from the initial placement at six months after surgery.5
Undesired toric IOL rotation can occur early or late in the postoperative period. A large degree of IOL rotation may occur in eyes with relatively long axial lengths, especially during the first few days post-op.6 The posterior capsule appears to adhere more tightly to the IOL within the first postoperative month.
For this reason, urgently refer patients with rotated IOLs back to the surgeon. Most surgeons request that the patient be returned within one to two weeks when toric IOL rotation is discovered.
 |
|
|
Toric IOL to posterior vault formation following surgical repositioning of the axis. The arrows point to the IOL axis markers.
|
Case #3: Peripheral Corneal Ulcer After Surgery
• History. A 53-year-old white female presented with a complaint of a “beet red” left eye and twinges of severe eye pain when moving the eye in a lateral direction over the past two months. She also complained of visual blur, whether focusing at distance or near. She was concerned that she had perhaps inadvertently rubbed facial moisturizer into her eye.
Three years earlier, this patient had undergone uncomplicated cataract surgery in her left eye. She had a history of multiple sensitivities to topical and systemic medications and was therefore anxious about any type of treatment for her condition.
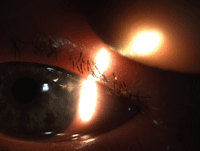
• Diagnostic data. Visual acuity in the left eye, which had previously been best-corrected to 20/25-, now measured 20/50 with pinhole. Biomicroscopy examination revealed a diffusely edematous cornea and an eroded limbal corneal suture at the large cataract incision. The site of the eroded suture was also deeply infiltrated with a 2+ cellular reaction in the anterior chamber. In addition, there was sectoral engorgement of conjunctival vasculature along with early corneal neovascularization pointing to the infiltrate.
• Diagnosis and management. This patient had a peripheral, sight-threatening corneal ulcer following cataract surgery in the left eye.
We needed to remove the suture. Because it was sight threatening, a same-day corneal culture with agar plating was initiated. The suture was placed in a thioglycolate broth tube and sent out to the lab.
We considered fortified antibiotics, but decided against them due to the patient’s history of sensitivities and allergies. Instead, we treated her every hour with AzaSite (azithromycin 1%, Akorn) and Zymaxid (gatifloxacin 0.5%, Allergan), both of which she had tolerated in the past.
The lab report came back the next day identifying Staphylococcus aureus, so we discontinued AzaSite. The ulcer proceeded to respond well to the topical fluoroquinolone treatment.
• Discussion. Most in-situ corneal sutures we encounter are monofilament nylon. This material has a characteristic black and shiny look. These sutures weaken with time but do not biodegrade. Sometimes we see sutures made of Vicryl (polyglactin, Ethicon), which are eventually absorbed by the body.
Knowing when and why the surgeon has used corneal sutures is the key to determining whether to remove them.
 | |
|
Eroded limbal suture resulting in infectious keratitis following cataract surgery.
|
Most of the time, postoperative cataract patients in our office have undergone clear-corneal incision cataract surgery, which usually does not require a suture. Occasionally, a surgeon reinforces one of these incisions with a small limbal stitch to ensure that the wound does not leak. Keep in mind that its presence does not indicate that there was a perioperative complication.
Common postsurgical practice supports removing these sutures at about one month after surgery. The sutures can remain in for longer, but most doctors recommend removal before three months.
Sutures that induce corneal astigmatism may prompt more immediate removal. Larger incisions or multiple interrupted sutures in one area may require multiple office visits for more strategic and delayed removal.
Eroded corneal sutures notoriously cause patient discomfort and often lead the patient to come in urgently. They should always be removed unless there is concern about an open globe. As this case demonstrates, untreated, eroded or broken sutures can lead to corneal ulcers and corneal neovascularization. This supports studies that claim short-term prophylactic antibiotic treatment is indicated following suture removal.7
Be sure to contact your surgeon if you have any doubt about when or how to extract such corneal sutures.
Postoperative care provides an engaging and rewarding opportunity to practice at the full scope of our profession. Critical to this care is a solid understanding of unforeseen medical complications with associated treatment regimens, as well as the ability to manage patient expectations.
For any sight-threatening medical postoperative complication, be sure to promptly notify the surgeon and refer the patient back. In addition, postoperative complexities regarding refractive visual outcomes should also be returned for an enhancement consultation.
Issues involving IOL malposition, when detected in the immediate postoperative period, are also considered to be urgent referrals. This offers the surgeon more flexibility with retreatment options and can ultimately lead to better patient outcomes with reduced chair time.
Dr. Jones practices at Northwest Eye Surgeons, a surgical subspecialty office in Seattle, WA. Dr. Talley Rostov is a partner and a cornea, cataract and refractive surgeon at Northwest Eye Surgeons.
1. Watson, A, Sunderraj P. Comparison of small-incision phacoemulsification with standard extracapsular cataract surgery: Postoperative astigmatism and visual recovery. Eye (Lond). 1992;6 (Pt 6):626-9.2. Mpyet C, Langnap L, Akpan S. Outcome and benefits of small incision cataract surgery in Jos, Nigeria. Niger J Clin Pract. 2007 Jun;10(2):162-5.
3. Mozayan E, Lee JK. Update on astigmatism management. Curr Opin Ophthalmol. 2014 Jul;25(4):286-90.
4. Novis C. Astigmatism and toric intraocular lenses. Curr Opin Ophthalmol. 2000 Feb;11(1):47-50.
5. Horn JD. Status of toric intraocular lenses. Curr Opin Ophthamol. 2007 Feb;18(1):58-61.
6. Miyake T, Kamiya K, Amano R, et al. Long-term clinical outcomes of toric intraocular lens implantation in cataract cases with preexisting astigmatism. J Cataract Refract Surg. 2014 Aug 20. [Epub ahead of print]
7. Heaven CJ, Davison CR, Cockcroft PM. Bacterial contamination of nylon corneal sutures. Eye (Lond). 1995;9(Pt 1):116-8.

