Non-arteritic anterior ischemic optic neuropathy (NAION) is a common condition that causes sudden, painless vision loss in older individuals. One possible etiology of NAION is acute blood loss that results in hypotension, anemia and associated optic nerve ischemia. The optic neuropathy that results from this sequence of events has been termed shock-induced anterior ischemic optic neuropathy (SIAION).1
Although rare, SIAION has been documented in patients who have suffered acute blood loss as well as a dramatic change in blood pressure secondary to systemic surgery.
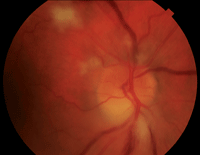
1. Fundus examination of the right eye revealed an edematous optic nerve with inferior pallor. We documented cotton-wool spots in the peripapillary region and one inferior splinter hemorrhage.
Here, we present the case of a patient who developed SIAION after undergoing complicated radical retropubic prostatectomy. Concurrently, the patient was found to have bilateral disc swelling, and was subsequently diagnosed with a frontal lobe meningioma. It is possible that the optic nerve swelling might have either precipitated or contributed to the development of AION.
History
A 61-year-old white male presented to the eye clinic complaining of a “purple haze” over the superior half of his vision O.D. after waking from a radical retropubic prostatectomy two weeks earlier. The patient also reported that he saw bilateral flashing lights when his eyes were closed. The symptoms had not changed since initial onset.
His medical history was significant for sleep apnea, colonic polyps, mixed hyperlipidemia, hypertension, allergic rhinitis and prostate cancer.
His preoperative blood pressure measured 154/94mm Hg, with a hemoglobin count of 15.2g/dL and a hematocrit volume of 45%. After surgery, his blood pressure measured 92/52mm Hg, his hemoglobin count was 9.1g/dL, and his hematocrit volume was 27%. The patient required six pints of A-negative blood due to significant perioperative and postoperative bleeding.
At the time of the patient’s first visit, his medical therapy consisted of belladonna/opium suppository (for severe pain from spasms of the urinary tract), ciprofloxacin, clonidine, diphenhydramine, docusate, hydrochlorothiazide, lisinopril, metoprolol, oxybutynin chloride, oxycodone/acetaminophen and promethazine injections.
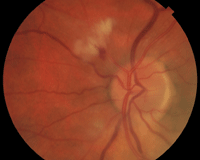
2. The left optic nerve optic nerve appeared
congested with indistinct margins. There were a few peripapillary cotton-wool spots as well as one small adjacent hemorrhage.
Diagnostic Data
His best-corrected visual acuity was 20/20 O.D. and O.S. Confrontation visual fields revealed a superior defect in the right eye. Additionally, we noted a 2+ relative afferent pupillary defect (RAPD) O.D.
His intraocular pressure was 15mm Hg O.U. Slit-lamp examination of the anterior segment was unremarkable. Fundus examination revealed an edematous, pale optic nerve in the right eye with one inferior splinter hemorrhage (figure 1). The left optic nerve appeared congested with indistinct margins (figure 2). Cotton-wool spots were present in the peripapillary region of both eyes (O.D. > O.S.). Humphrey visual field testing revealed a complete superior altitudinal defect in the right eye, as well as blind spot enlargement in the left eye (figure 3).
The pale, swollen optic nerve, RAPD and superior altitudinal visual field defect in the right eye were indicative of ischemic optic neuropathy, which was likely related to blood loss sustained during surgery. The cause of the left optic nerve swelling was not as clear, so we ordered an MRI.
The MRI revealed a right frontal lobe lesion with marked surrounding edema (figures 4 and 5). The lesion measured approximately 1.5cm x 1.7cm x 1.8cm, with a large area of surrounding edema that extended back to compress the fluid space surrounding the right optic nerve (figures 6 and 7). We noted significant mass effect, with a right-to-left midline shift of approximately 5mm.
Diagnosis
The patient’s optic nerve appearance, in conjunction with his medical history and symptoms, led us to a diagnosis of SIAION in the right eye. Based on the clinical appearance and the presence of an enlarged blind spot on visual field testing, we determined papilledema to be the cause of disc swelling in the left eye. Papilledema likely contributed to the development of ischemic optic neuropathy in his right eye.
Treatment and Follow-up
Neurology started the patient on dexamethasone for edema and levetiracetam for seizure prophylaxis. The patient was scheduled for a right frontal craniotomy the following week.
Right frontal craniotomy with resection of a 2.5cm x 2.0cm x 1.3cm pink/tan nodule was performed successfully. Biopsy of the mass revealed a psammomatous-type meningioma. The patient informed his neurologist that his vision was unchanged following the procedure.
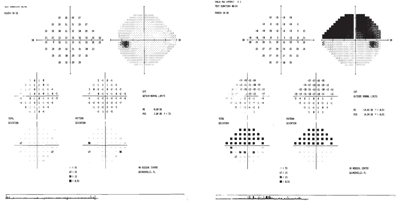
3. Humphrey visual field testing revealed a complete superior altitudinal defect in the right eye and slight blind spot enlargement in the left eye.
Two weeks after the craniotomy, the patient was examined in the eye clinic and exhibited similar diagnostic findings to those obtained at the initial visit. Humphrey visual field testing showed a persistent––but stable––complete superior altitudinal defect in the right eye, with a full field in the left eye.
Three months after his initial visit, the patient’s best-corrected visual acuity remained 20/20 O.U. The superior defect in the right eye was stable on confrontation fields testing, as was the 2+ RAPD. Fundus examination showed that the bilateral optic disc edema, hemorrhages and cotton-wool spots had resolved. Additionally, the right optic nerve appeared pale inferiorly while the left optic nerve looked healthy and pink.
The patient was in good spirits and reported an excellent recovery from the craniotomy. Although the condition was stable at this visit, the patient was considered to be at risk for AION in the fellow eye secondary to a small cup-to-disc ratio. We advised the patient to avoid taking antihypertensive medications at bedtime, to continue daily aspirin therapy as prescribed by his primary care provider, and to schedule a follow-up appointment in six months.
Discussion
NAION is an acute ischemic disorder of the optic nerve that occurs at the most anterior portion of the optic nerve.2 This painless event is characterized by sudden vision loss and visual field defects that vary in both severity and pattern. It is one of the most common non-reversible causes of vision loss in middle-aged and elderly individuals; in 14.7% of patients, the second eye becomes involved within about five years.3,4
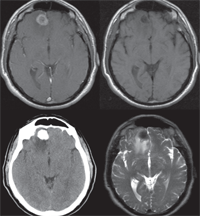
4. Axial images of the tumor with different imaging strategies. T1-weighted MRI with Gadolinium (top left). T1-weighted MRI without contrast (top right). Non-contrast CT scan (bottom left). T2-weighted MRI (bottom right).
Clinically, patients with NAION present with a visual acuity that ranges from better than 20/20 to no light perception.5 Usually, patients also exhibit a RAPD unless the disease is bilateral and symmetric (which is rare). Visual field defects in these patients vary––with superior and inferior arcuates being the most prevalent––and often remain relatively stable after six months.6 Initial funduscopic appearance always includes optic disc edema, which may be more prominent in one region (sectoral). Frequently, splinter hemorrhages are seen at the disc margin.7 Cotton-wool spots may be present in the peripapillary region of the affected eye. The initial presentation is followed by a gradual transition from optic disc edema to pallor.
• Risk factors. Multiple predisposing systemic and ocular risk factors have been identified in the development of NAION. Systemic risk factors include diabetes, collagen vascular diseases, malignant hypertension, hypotension, hyperlipidemia, arteriosclerosis, massive recurrent systemic hemorrhages, migraine and other vasospastic disorders, hematologic disorders, cardiac valvular disease, defective cardiovascular autoregulation, type A behavior pattern, sleep apnea and internal carotid artery disease.6,8-10
Ocular conditions that have been associated with NAION include an absent or small cup in the optic disc, optic disc drusen, elevated intraocular pressure, marked optic disc edema due to any cause, location of the watershed zone of the posterior ciliary arteries within the optic disc and vascular disorders located in the nutrient vessels of the optic nerve head.11-15
Another cause of NAION is severe blood loss secondary to systemic, non-ocular surgical procedures or significant trauma that results in systemic hypotension and anemia.1 In such cases, the condition is often referred to as SIAION. Perioperative risk factors for the development of SIAION include prolonged surgical duration, acute systemic hypotension, anemia due to blood loss or prone positioning.16 Rarely, SIAION may involve both optic nerves, resulting in bilateral vision loss.9 Underlying systemic and ocular risk factors may further predispose a patient to the development of SIAION either during or after surgery.
• Incidence and etiology. Incidence of SIAION varies with type of systemic surgery and/or surgical location site. One retrospective study indicated that one in 125,234 non-cardiac surgeries resulted in prolonged vision loss without direct surgical trauma to the orbit or cerebral tissues.17 This incidence is relatively low when compared to a study of cardiac surgeries in which 22 of 312 patients developed visual field loss or a reduction in visual acuity.18
NAION is caused by transient nonperfusion or hypoperfusion of the paraoptic branches of the posterior ciliary artery circulation within the optic nerve head.19,20 Blood flow within the optic nerve head is dependent upon perfusion pressure (mean blood pressure minus intraocular pressure) divided by the resistance to blood flow. Resistance to blood flow is determined by the state and caliber of the blood vessels as well as the viscosity of the individual’s blood.10
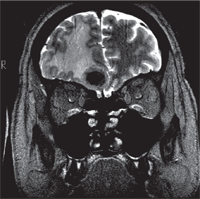
5. Coronal T2-weighted MRI showing frontal lobe edema and mid-line shift.
Blood flow within the optic nerve head vessels normally is autoregulated, with a goal of relatively constant blood flow during changes in perfusion pressure.15,21 Autoregulation operates over a critical range of perfusion pressures. If the perfusion pressure falls outside this critical range, autoregulation breaks down and hypoperfusion or transient nonperfusion may result in NAION.10 Decreased perfusion pressure can be caused by a marked reduction in mean blood pressure secondary to shock, nocturnal hypotension, severe internal carotid artery disease, or ophthalmic artery stenosis or occlusion.22 An increase in intraocular pressure or a combination of decreased mean blood pressure and increased intraocular pressure also can reduce perfusion pressure.23
Chronic arterial hypertension is thought to cause derangement of the blood flow autoregulation mechanism. This occurs when the critical range is shifted to a higher level in an attempt to compensate for the higher mean blood pressure. This shift permits sufficient tolerance for hypertension but results in decreased tolerance for hypotension, resulting in an autoregulatory breakdown in the event of sudden blood pressure drop. This, in turn, leads to decreased blood flow to the optic nerve, resulting in ischemia and possible NAION.15
The aforementioned scenario often occurs during nocturnal arterial hypotension in patients who are using intensive antihypertensive medication, and is thought to be the pathogenesis of the common NAION symptom of vision loss upon waking.15
Less commonly, massive blood loss or shock can be the cause of a sudden blood pressure drop.3 A 24% to 46% decrease in postoperative blood pressure from preoperative levels that occurs within 15 minutes to two hours after the surgical procedure has been reported in patients who developed SIAION.24
In the case of massive blood loss, a decrease in hemoglobin creates a lack of oxygen-carrying capacity in the blood, which further compounds ischemia of the optic nerve.9 Anemia and low plasma proteins after hemorrhage are thought to create low oncotic pressure, which results in ischemia and vessel wall damage.9 A small, retrospective study of six patients with acute blood loss who subsequently developed SIAION showed significant anemia with hemoglobin levels less than 8g/dL (reference range: 13.9g/dL to 18g/dL) from 30 minutes to 72 hours after surgery.24
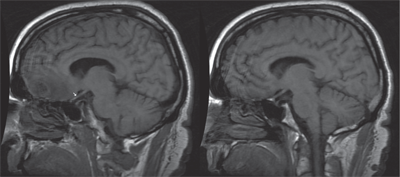
6. Sagittal T1-weighted MRI image (left) shows edema extending posteriorly from the tumor and impinging superiorly on the right optic nerve (see arrow). The left eye shows preservation of the space located superior to the optic nerve.
Blood flow resistance can be affected by vascular changes in the small blood vessels within the optic nerve head and/or the arteries that feed optic nerve circulation. These vascular changes may be caused by vasospasm, arteriosclerosis, atherosclerosis, vasculitis, drug-induced vasoconstriction/dilation, or other cardiovascular and systemic diseases.10 Studies also have shown that swollen axons in the restricted space within the optic disc produce secondary vascular changes by compressing the capillaries and other fine vessels located along the nerve fiber bundles.25
• Management strategies. Currently, there is no known treatment for NAION––although many treatments have been recommended. Therapeutic attempts to improve visual acuity or prevent contralateral eye involvement range from noninvasive topical and oral
therapies to optic nerve decompression surgery. Aspirin therapy, although often suggested to prevent fellow eye involvement, was found to have no effect on the second eye five to eight years after initial occurrence.4,26 Brimonidine tartrate also has been studied for its potential neuroprotective effect. However, clinical trials have failed to yield clinical efficacy in humans.27
Even the most well known study related to NAION––the Ischemic Optic Neuropathy Decompression Trial––eventually showed no visual benefit from interventional treatments and was abandoned early due to poor outcomes.6 Indeed, subjects in the control group fared better than those in the surgery group.
Another invasive therapy is anti-VEGF injection. In one study of three patients who received an intravitreal ranibizumab injection one to two days after the onset of NAION, the researchers documented a reduction in optic nerve swelling, but no functional improvement.28
Additionally, a case control study of 696 eyes showed promising results when oral corticosteroid therapy was initiated after the onset of NAION.29 Subjects were treated with 80mg of oral prednisone for two weeks, which was then tapered every five days over a two- to three-month period. Oral steroid treatment yielded greater improvement in visual acuity and visual field testing when compared to the control group over a three- to nine-month period. Significant change in visual acuity was quantified as three lines on a Snellen chart (0.3 logMAR). Visual fields improved in 40.1% of the treated group compared to 24.5% of the untreated group. Improvement was more substantial in subjects whose entering visual acuities measured 20/70 or worse.29,30
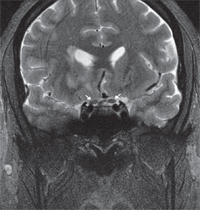
7. Coronal T2-weighted MRI showing the right optic nerve slightly compressed/distorted due to mass effect from the edema surrounding and extending from the tumor (see arrows).
• Papilledema and Foster-Kennedy syndrome. Papilledema refers to bilateral optic disc swelling secondary to elevated intracranial pressure. Some of the more common causes of increased intracranial pressure include space-occupying lesions, aqueductal stenosis that produces hydrocephalus, pseudotumor cerebri, subdural or epidural hematomas, subarachnoid hemorrhage, arteriovenous malformations, meningitis, encephalitis and intracranial venous sinus thrombosis.31
In our patient’s case, a space-occupying psammomatous meningioma was suspected to be causing increased intracranial pressure, which led to disc swelling in the left eye. This finding would also cause the symptom of flashing lights with no acute vision loss (as in the right eye), as well as the presence of an enlarged blind spot in the left visual field. This type of benign meningioma is classified by dense epithelial clusters of tumor cells that form numerous corpuscles (which resemble the layers of an onion), leading to calcification.32
Although the pathogenesis of papilledema is still unclear, multiple theories have been suggested. These include, but are not limited to, direct infiltration of the optic nerve head by cerebrospinal fluid, turgescence of prelaminar optic nerve tissue, glial edema, disturbance of hydrostatic pressure in the nerve tissue and blood stream, and compression of the central retinal vein with elevated venous pressure.33-37 However, it is clear that axoplasmic transport is impaired with papilledema.38 As with all forms of optic disc swelling, there is blockage of both slow and fast axoplasmic transport, with subsequent axoplasmic backflow.39
Optic disc swelling occurs in steps, with the earliest sign being blurring of the disc margin due to swelling of the axons in the peripapillary nerve fiber layer.13,40 Blurring begins in the lower pole, then includes the upper pole and nasal border, with the temporal margin blurring last.41 Hyperemia occurs next, and is caused by dilation of the capillaries located within the disc.40,41 Also, loss of spontaneous venous pulse (if present) occurs at this stage.42 Further, disc elevation is seen in advanced cases of disc swelling, starting in the periphery and moving centrally.43 Other common features of papilledema include radially oriented splinter hemorrhages in the peripapillary nerve fiber layer, exudates and cotton-wool spots.43
Papilledema typically does not result in vision loss in its early stages. However, if left untreated, visual field defects may occur––with enlargement of the physiological blind spot being the earliest (and often the only) defect. The defects can progress, and eventually might lead to severe visual field constriction or central vision loss.43 Transient visual obscurations, including photopsia, have also been reported by patients with papilledema.44,45 The cause and clinical significance of this phenomenon remains unknown.46
Our patient reported this unusual symptom on presentation, specifically noticing the flashing lights when he had his eyes closed. Although uncommon, superimposed ischemic optic neuropathy can result from severely swollen nerves. This particular phenomenon has been seen in patients with underlying papilledema and hypertension who subsequently suffered acute hypotension.47 This might further explain our patient’s clinical presentation.47
No discussion on papilledema would be complete without mention of Foster-Kennedy syndrome (FKS)––a condition that is defined by the presence of papilledema in one eye and optic disc atrophy in the fellow eye secondary to an intracranial mass. The incidence of FKS has been shown to be less than 1% in conjunction with intracranial tumors.48
When a patient presents with these clinical signs in the absence of a compressive tumor, the condition is often termed pseudo-Foster-Kennedy syndrome (PFKS). Mimicking conditions include bilateral non-simultaneous optic neuritis or ischemic optic neuropathy, papillitis with an old optic neuropathy, and papilledema with an old optic neuropathy.49
However, our patient experienced what appeared to be AION in one eye and optic disc swelling in the fellow eye––a presentation inconsistent with either FKS or PFKS.
SIAION is a rare but potentially visually devastating consequence of surgical or traumatic blood loss that may be exacerbated by an underlying systemic disease. In this case, our middle-aged patient with pre-existing hypertension and hyperlipidemia underwent a complicated, non-ocular surgical procedure and experienced significant blood loss that resulted in systemic hypotension and anemia. As a result, he developed an acute and permanent loss of vision in his right eye due to SIAION. Unknown to the patient or the medical professionals who were initially involved in his care, he also had a right frontal lobe meningioma.
Upon initial fundus examination, we observed bilateral asymmetric disc edema with splinter hemorrhages and cotton-wool spots. The disc edema in the non-symptomatic eye led to the discovery of the meningioma on MRI. We believe that the edema surrounding the frontal lobe menigioma likely played a role in predisposing this patient to NAION in the right eye, as there is evidence that axonal swelling from optic disc edema caused increased blood flow resistance that led to further ischemia.
Whether the optic disc swelling in the fellow eye represented papilledema or atypical/mild SIAION remains unknown. The lack of acute SIAION symptomology, the perception of light flashes and the presence of an enlarged blind spot on visual field testing suggest papilledema as the likely cause of optic disc swelling in the left eye. This finding ultimately led to further investigation and subsequent discovery of the meningioma.
Regardless of the role papilledema may have played, this case taught us that patients can––and sometimes do––suffer simultaneous, coincidental ocular disorders. Even though SIAION was the obvious suspect in our patient, this case reminds us that every patient with bilateral disc edema requires neuroimaging.
Dr. Perkins is a staff optometrist at The Villages VA Outpatient Clinic in Florida. Dr. Marcus-Freeman is a staff optometrist and optometry residency coordinator at the Malcom Randall VA Medical Center.
1. Foroozan R, Buono LM, Savino PJ. Optic disc structure and shock-induced anterior ischemic optic neuropathy. Ophthalmology. 2003 Feb;110(2):327-31.
2. Hayreh SS. Anterior ischemic optic neuropathy-I. Terminology and pathogenesis. Br J Ophthalmol. 1974 Dec;58(12):955-63.
3. Hayreh SS, Bill A, Sperber GO. Effects of high intraocular pressure on the glucose metabolism in the retina and optic nerve in old atherosclerotic monkeys. Graefes Arch Clin Exp Ophthalmol. 1994 Dec;232(12):745-52.
4. Newman N, Scherer R, Langenberg P, et al. The fellow eye in NAION: Report from the Ischemic Optic Neuropathy Decompression Trial follow-up study. Am J Ophthalmol. 2002 Sep;134(3):317-28.
5. Hayreh SS, Podhajsky P. Visual field defects in anterior ischemic optic neuropathy. Doc Ophthalmol Proc. 1979;19:53-71.
6. Newman N. The ischemic optic neuropathy decompression trial. Arch Ophthalmol. 2007 Nov;125(11):1568-70.
7. Hayreh SS. Anterior ischemic optic neuropathy. II. Fundus on ophthalmoscopy and fluorescein angiography. Br J Ophthalmol. 1974 Dec;58(12):964-80.
8. Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J J Neuroophthalmol. 2003 Jun;23(2):157-63.
9. Hayreh SS. Anterior ischemic optic neuropathy VIII. Clinical features and pathogenesis of post-hemorrhagic amaurosis. Ophthalmology. 1987 Nov;94(11):1488-502.
10. Hayreh SS. Anterior ischemic optic neuropathy. Clin Neurosci. 1997;4(5):251-63.
11. Beck RW, Sevais GE, Hayreh SS. Anterior ischemic optic neuropathy – IX. Cup-to-disc ratio and its role in pathogenesis. Ophthalmology. 1987 Nov;94(11):1503-8.
12. Purvin V, King R, Kawasaki A, Yee R. Anterior ischemic optic neuropathy in eyes with optic disc drusen. Arch Ophthalmol. 2004 Jan;122(1):48-53.
13. Hayreh SS. Optic disc edema in raised intracranial pressure. VI. Associated visual disturbances and their pathogenesis. Arch Ophthalmol. 1977 Sep;95(9):1566-79.
14. Hayreh SS. Inter-individual variation in blood supply to the optic nerve head. Its importance in various ischemic disorders of the optic nerve head, and glaucoma, low-tension glaucoma and allied disorders. Doc Ophthalmol. 1985 Apr 30;59(3):217-46.
15. Hayreh SS. The optic nerve head circulation in health and disease. Exp Eye Res. 1995 Sep;61(3):259-72.
16. Katz DM, Trobe JD, Cornblath WT, Kline LB. Ischemic optic neuropathy after lumbar spine surgery. Arch Ophthalmol. 1994 Jul;112(7):925-31.
17. Warner ME, Warner MA, Garrity JA, et al. The frequency of perioperative vision loss. Anesth Analg. 2001 Dec;93(6):1417-21.
18. Shaw PJ, Bates D, Cartlidge NE, et al. Neurologic and neuropsychological morbidity following major surgery: comparison of coronary artery bypass and peripheral vascular surgery. Stroke. 1987 Jul-Aug;18(4):700-7.
19. Hayreh SS. Anterior ischemic optic neuropathy. Arch Neurol. 1981 Nov;38(11):675-8.
20. Arnold A, Hepler R. Fluorescein angiography in acute nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1994 Feb 15;117(2):222-30.
21. Geijer C, Bill A: Effects of raised intraocular pressure on retinal, prelaminar, laminar, and retrolaminar optic nerve blood flow in monkeys. Invest Ophthalmol Vis Sci. 1979 Oct;18(10):1030-42.
22. Hayreh SS, Podhajsky P. Ocular neovascularization with retinal vascular occlusion. II. Occurrence in central and branch retinal artery occlusion. Arch Ophthalmol. 1982 Oct;100(10):1585-96.
23. Hayreh SS. Anterior ischemic optic neuropathy. IV. Occurrence after cataract extraction. Arch Ophthalmol. 1980 Aug;98(8):1410-6.
24. Brown RH, Schauble JF, Miller NR. Anemia and hypotension as contributors to perioperative loss of vision. Anesthesiology. 1994 Jan;80(1):222-6.
25. Mcleoad D, Marshall J. Role of axoplasmic transport in the pathophysiology of ischemic disc swelling. Br J Ophthalmol. 1980 Apr;64(4):247-61.
26. Hart RG, Halperin JL, McBride R, et al. Aspirin for primary prevention of stroke and other major vascular events: meta-analysis and hypotheses. Arch Neurol. 2000 Mar;57(3):326-32.
27. Saylor, M, McLoon LK, Harrison AR, Lee MS. Experimental and clinical evidence for brimonidine as an optic nerve and retinal neuroprotective agent. Arch Ophthalmol. 2009 Apr;127(4):402-6.
28. Pece A, Querques G, Quinto A, Isola V. Intravitreal ranibizumab injection for nonarteritic ischemic optic neuropathy. J Ocul Pharmacol Ther. 2010 Oct;26(5):523-7.
29. Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy: role of systemic corticosteroid therapy. Graefes Arch Clin Exp Ophthalmol. 2008 Jul;246(7):1029-46.
30. Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res. 2009 Jan;28(1):34-62
31. Kunimoto DY, Kanikar KD, Makar MS, et al. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. Philadelphia: Lippincott, Williams and Wilkins; 2004.
32. Riede UN, Werner M. Color atlas of pathology: pathologic principles, associated diseases, sequel. New York: Thieme; 2004.
33. Fry WE. The pathology of papilledema: an examination of 40 eyes with special references to compression of the central retinal vein. Am J Ophthalmol. 1931;14:874-83.
34. Van Heuven JA, Fischer PF. The water binding of the optic nerve and its sheaths. Br J Ophthalmol. 1936 Apr;20(4):204-13.
35. Rosomoff HL, Zugibe FT. Distribution of intracranial contents in experimental edema. Arch Neurol. 1963 Jul;9:26-34.
36. Hedges TR, Weinstein JD. The hydrostatic mechanism of papilledema. Trans Am Acad Ophthalmol Otolaryngol. 1968 Sep-Oct;72(5):741-50.
37. Sanders MD. The Bowman Lecture. Papillodema: the pendulum of progress. Eye (Lond). 1997;11(Pt 3):267-94.
38. Taylor AC, Weiss P. Demonstration of axonal flow by movement of tritium-labeled protein in mature optic nerve fibers. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1521-7.
39. Wirtschafter JD, Rizzo FJ, Smiley C. Optic nerve axoplasm and papilledema. Surv Ophthalmol. 1975 Nov-Dec;20(3):157-89.
40. Frisen L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982 Jan;45(1):13-8.
41. Digre KB, Corbett JJ. Practical Viewing of the Optic Disc. Burlington, Vt.: Butterworth-Heinemann; 2004.
42. Levin BE. The clinical significance of spontaneous pulsations of the retinal vein. Arch Neurol. 1978 Jan;35(1):37-40
43. Van Stavern G. Optic disc edema. Semin Neurol. 2007 Jul;27(3):233-43.
44. Newman B. Neuro-Ophthalmology Illustrated. New York: Thieme; 2009.
45. Giuseffi V, Wall M, Siegel PZ, Rojas PB. Symptoms and disease associations in idiopathic intracranial hypertension (pseudotumor cerebri): a case-control study. Neurology. 1991 Feb;41(2(Pt 1)):239-44.
46. Schirmer C, Hedges T. Mechanisms of Visual Loss in Papilledema. Neurosurg Focus. 2007;23(5):E5.
47. Green GJ, Lessell S, Loewenstein JL. Ischemic optic neuropathy in chronic papilledema. Arch Ophthalmol. 1980 Mar;98(3):502-4.
48. Lotifipour S, Chiles K, Kahn JA. An unusual presentation of subfrontal meningioma: a case report and literature review for Foster Kennedy syndrome. Intern Emerg Med. 2011 Jun;6(3):267-9.
49. Limaye SR, Adler J. Pseudo-Foster Kennedy syndrome in a patient with anterior ischemic optic neuropathy and a nonbasal glioma. J Clin Neuroophthalmol. 1990 Sep;10(3):188-92.

