Corneal endotheliopathy is a broad term used to classify several diseases and clinical circumstances that affect the structure and function of the corneal endothelium. Because the endothelium is responsible for maintaining proper corneal hydration, clinically significant corneal endotheliopathies can lead to edema and a loss of transparency.1
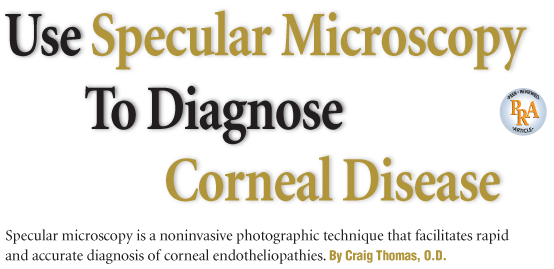
1. Factors that can compromise the corneal endothelium and cause corneal edema.3
Common ocular conditions, such as glaucoma, uveitis and Fuchs endothelial dystrophy, may produce changes in the structure and function of the corneal endothelium that result in corneal edema and visual impairment. Additionally, clinical circumstances, such as contact lens wear and intraocular surgery, may compromise the endothelium and cause corneal edema (figure 1). An accurate diagnosis of endothelial disease may be the key in not only determining cause of corneal edema, but also developing a treatment plan.
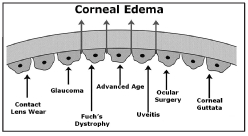
Specular microscopy is a noninvasive photographic technique that allows you to visualize and analyze the corneal endothelium. Using computer-assisted morphometry, modern specular microscopes analyze the size, shape and population of the endothelial cells. The instrument projects light onto the cornea and captures the image that is reflected from the optical interface between the corneal endothelium and the aqueous humor. The reflected image is analyzed by the instrument and displayed as a specular photomicrograph (figure 2). In clinical practice, specular microscopy is the most accurate way to examine the corneal endothelium.
2. Specular photomicrograph of a normal corneal endothelium in a 21-year-old female patient.
The Corneal Endothelium
The corneal endothelium is a monolayer of 350,000 to 500,000 specialized cells that cover the posterior surface of the cornea. One of the endotheliums physiological functions is to secrete a collagen matrix that forms Descemets membrane.1 But, the primary physiological function of the corneal endothelium is to maintain the health and transparency of the corneal stroma. Because the cornea is avascular, the supply of nutrients occurs via diffusion of glucose and other solutes from the anterior chamber across the cornea endothelium. To facilitate diffusion, intraocular pressure constantly forces aqueous into the stroma from the anterior chamber. Although the influx of aqueous into the stroma is necessary to maintain corneal health, the level of corneal hydration must be controlled to prevent edema.
Biomechanical properties of the cornea. Corneal hydration is one of the biomechanical properties of the anterior segment, and it is affected by several interdependent factors.2 To control corneal hydration, the endothelium forms an anatomical and physiological barrier to the aqueous that makes the structure semi-permeable.
Fluid barrier function. Endothelial permeability is controlled by tight junctions that are formed between endothelial cells. These connections between the cells are found at the apex of the lateral cell membrane and serve to restrict the amount of fluid entering the corneal stroma.3
Metabolic pump function. In addition to its barrier function, the endothelium also maintains stromal deturgescence by pumping fluid out of the stroma through an active transport mechanism. The site of the metabolic pump is also within the lateral cell membrane, and it is a part of a completely formed junctional complex between the endothelial cells. The active transport pumping mechanism uses enzymes to translocate bicarbonate ions across the endothelial cell membrane, which passively permits water to follow the ions into the anterior chamber.4
Normal Corneal Endothelium
With specular microscopy, the corneal endothelium appears as a somewhat-regular array of cellsthe endothelial mosaic. In this mosaic configuration, all of the endothelial cells appear to be approximately the same size and shape.5
Stability of the endothelial mosaic. In a normal endothelium, more than 60% of the endothelial cells are six-sided. The size and shape of the endothelial cells is important because adjacent cells with similar dimensions best maintain the fluid barrier function of the endothelium.6
Rate of polymegethism. Complete coverage of the posterior corneal surface is required to maintain the barrier function and the active transport mechanism of the corneal endothelium. Because of normal attrition, the central cornea loses 100 to 500 endothelial cells per year. When these cells die, they slough off the posterior surface of the cornea into the anterior chamber, creating a gap in the endothelial mosaic that compromises both the barrier and pump functions of the endothelium.
To repair the gap, the endothelium relies on cellular migration and cellular fusion.3 In this wound repair mechanism, the endothelial cells adjacent to the defect move to fill in the space vacated by the sloughed cell. The endothelial cells either stretch and slide into a different position, or they fuse together to re-establish complete coverage of the posterior surface of the cornea.3 This movement of the endothelial cells creates a variation in cell size known as polymegethism.
Because polymegethism is a reflection of the normal endothelial cell movement that characterizes the wound repair mechanism, there is always some degree of polymegethism in the corneal endothelium. The rate of polymegethism is represented by the coefficient of variation (CV). CV values measured between 0.22 and 0.31 are considered normal.7
Endothelial cell density. Maintaining both the barrier and pump function requires a certain number of endothelial cells to cover the posterior surface of the cornea. The minimum number of cells, or critical cell density, averages between 300 and 500 cells/mm2.4,8
The central corneal endothelium changes as a person ages. From age 20 to 50, the endothelial cell density remains relatively stable for most people. Beyond age 50, a slow decline begins. By age 60, most people experience a significant reduction in endothelial cell density (see Endothelial Cell Density by Age).3,5,9
1,500 - 2,300
Endothelial Cell Density by Age
Age
Average Endothelial
Cell Density (cells/mm2)
10 - 19
2,900 - 3,500
20 - 29
2,600 - 3,400
30 - 39
2,400 - 3,200
40 - 49
2,300 - 3,100
50 - 59
2,100 - 2,900
60 - 69
2,000 - 2,800
70 - 79
1,800 - 2,600
80 - 89
Using specular microscopy, endothelial disease may be characterized by one or more abnormalities of cell morphology.
Presence of pleomorphism. Pleomorphism is a significant disruption in the regular hexagonal pattern of the endothelium that causes a decrease in endothelial mosaic stability. Pleomorphism occurs secondary to physiological stress from ocular disease, contact lens wear or normal aging changes.4,6,7
If a patients corneal endothelium demonstrates less than 50% hexagonally-shaped cells, he or she is considered to have clinically significant pleomorphism. Because of its effect on the fluid barrier function of the endothelium, the presence of pleomorphism increases the patients risk of developing iatrogenic corneal endotheliopathy and postoperative corneal edema.5
Elevated or abnormal rate of polymegethism. An elevated or abnormal rate of polymegethism is usually the first sign of endothelial disease. This finding indicates physiological stress to the corneal endothelium and an overactive wound repair mechanism. CV values from 0.32 to 0.40 are elevated, and CV values above 0.40 are abnormal. Although endothelial function may still be adequate in these corneas, the endothelium may be more susceptible to additional trauma from insults, such as intraocular surgery, glaucoma, diabetes, uveitis or contact lens wear.5
Abnormal reduction in endothelial cell density. Advanced age, disease and injury may produce significant reductions in endothelial cell density. When present, endothelial cell loss should be bilaterally symmetrical; a difference of more than 280 cells/mm2 is clinically significant.5
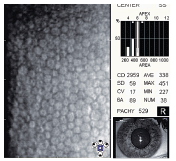
The appearance, enlargement or coalescence of corneal guttata. Corneal guttata are secretions of collagen from the endothelial cells that form a nodularity on the posterior surface of Descemets membrane (figure 3). These nodules are created when endothelial cells under physiological stress secrete an altered basement membrane material that accumulates under the cells. The deposits of abnormal collagen eventually form a nodular-shaped lesion called a corneal gutta.
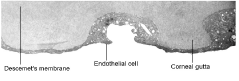
3. Transmission electron micrograph of corneal guttata. Endothelial cells wrap around and cover the corneal guttata to maintain complete coverage of the posterior corneal surface.3
Corneal Endotheliopathies
Corneal endotheliopathies are classified as primary or secondary. In primary corneal endotheliopathies, endothelial damage is not associated with any other ocular or systemic disorder. In secondary corneal endotheliopathies, there is a recognizable ocular or systemic disorder that contributes to the loss of endothelial cell structure and function. Examples of secondary endotheliopathies include contact lens-induced endotheliopathy, iatrogenic endotheliopathy and corneal endotheliopathy secondary to ocular inflammation.1
Corneal guttata. Corneal guttata are the most common primary corneal endotheliopathy. The clinical finding of corneal guttata is not specific; they may occur as part of the normal aging process, in corneal endothelial dystrophies, or secondary to ocular inflammation and trauma. Corneal guttata mainly affect the central region of the cornea and, when mild or moderate in presentation, they usually have no effect on visual acuity.10 They are present in 70% of the population over 40 years old.11
Corneal guttata can be visualized during biomicroscopy with the specular reflection illumination technique (figure 4). Under specular microscopy, a corneal gutta appears as a darkened area that resembles a hole in the endothelial mosaic (figure 5). The darkened area is created on the specular photomicrograph because the apex of the corneal gutta lies outside the specular reflections plane of focus.12

4. Corneal guttata visualized during a biomicroscopic examination.
The natural history of corneal guttata progression includes five specific stages of development, which can be discerned with specular microscopy. In stage 1, the gutta nodule appears as a small, dark structure in the center of an endothelial cell. In stage 2, the gutta nodule is almost the same size as an endothelial cell and the surrounding cells have a stretched appearance. In stage 3, the gutta is very large and many endothelial cells are involved with one nodule. Also, endothelial cells that are adjacent to large gutta have missing cell boundaries, and multiple guttae may be present. In stage 4, the individual gutta have coalesced and the endothelial cells between the guttata have become abnormal. In stage 5, progressive coalescence of corneal guttata continues, making the normal tessellation of the endothelial mosaic difficult or impossible to visualize.13
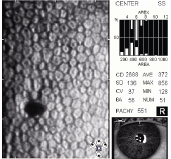
5. Specular photomicrograph of a corneal gutta.
Fuchs endothelial dystrophy. When confluent corneal guttata are present with clinically significant corneal edema, the condition is called Fuchs endothelial dystrophy (figure 6). This disease affects 4% of the population over 40 years old and is characterized by a progressive loss of endothelial cell structure and function. It eventually leads to corneal edema and a loss of corneal transparency.14
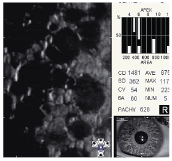
6. Confluent corneal guttata in Fuchs endothelial dystrophy.
Age-related endotheliopathy. Advancing age may produce abnormal or asymmetric reductions in endothelial cell density, an abnormal increase in the rate of polymegethism, clinically significant pleomorphism and/or an increase in the development of corneal guttata.5
Iatrogenic endotheliopathy. Surgical trauma during normal cataract surgery generally results in a 4% to 10% loss of endothelial cells. This postoperative cell loss is called iatrogenic endotheliopathy. In addition to the immediate cell loss associated with the procedure, cataract surgery also produces an accelerated rate of endothelial cell loss for at least 10 years after surgery. When too many cells are lost from iatrogenic endotheliopathy, endothelial function is compromised and postoperative corneal edema develops.1,4,7,15
Risk factors for the development of clinically significant iatrogenic endotheliopathy and postoperative corneal edema include previous ocular surgery, diabetes, glaucoma, previous ocular inflammation, an abnormal rate of polymegethism, the presence of pleomorphism, the presence of severe corneal guttata and Fuchs endothelial dystrophy.5,6,8,11 In patients with clinically significant iatrogenic endotheliopathy, specular microscopy reveals large reductions in endothelial cell density that occur over short periods of time.
7. Endothelium of a 55-year-old woman with a 30-year history of PMMA and gas-permeable contact lens wear. Note the abnormal rate of polymegethism, the presence of pleomorphism, the abnormal reduction in cell density and the presence of stages 1-3 corneal guttata.
Contact lens-induced endotheliopathy. Contact lens wear produces both acute and chronic morphologic changes in the corneal endothelium. Specifically, both the presence and rate of clinically significant polymegethism increases as the span of contact lens wear increases (figure 7).1,5-7,16,17
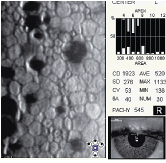
Although several mechanisms contribute to the development of contact lens-induced endotheliopathy, wearing contact lenses with low oxygen permeability appears to be the primary cause. Also, contact lens-induced hypoxia can produce endothelial cell damage and a loss of endothelial function secondary to chronic corneal swelling and de-swelling.5,6,16,17
When patients develop contact lens-induced endotheliopathy, discontinuing contact lens wear does not produce a rapid reversal of the morphologic changes. However, some degree of recovery is possible over several years if contact lens wear is discontinued, or if the patient switches to a contact lens with a significantly higher degree of oxygen permeability.6,7,16,17
As with all types of corneal endotheliopathy, contact lens induced-endotheliopathy may produce the clinical signs and symptoms associated with corneal edema. In addition to blurred vision, fluctuating vision and photophobia, contact lens wearers with clinically significant contact lens-induced endotheliopathy may complain of foreign body sensation when wearing their contact lenses, or corneal exhaustion syndrome in severe cases.6
Endotheliopathy secondary to ocular inflammation. Iridocyclitis can result in endothelial cell loss and reduced endothelial cell function.4 Specifically, iridocyclitis causes a release of immune response proteins into the anterior chamber that leads to endothelial cell death.18
8. Endothelium of a patient with acute anterior uveitis. Note the numerous dark structures and the dislodged endothelial cells in the center of the image.
Also, during an acute inflammatory episode, mononuclear inflammatory cells can penetrate the tight junctions between the endothelial cells and insert themselves between the endothelium and Descemets membrane. This can cause individual endothelial cells to dislodge and float free in the aqueous.5,17 In acute anterior uveitis, specular microscopy may reveal several well-demarcated dark structures usually located at endothelial cell intersections that are thought to be invading white blood cells (figure 8).5
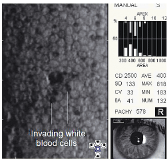
Glaucoma-induced endotheliopathy. Long-term exposure to elevated intraocular pressure can produce an abnormal reduction in endothelial cell density. One study suggested that the mechanism of cell loss is not the result of increased intraocular pressure, but rather some other physiological alteration in the glaucomatous eye, such as abnormal aqueous outflow or decreased oxygen concentration in the aqueous.5
Treatment Options
Although no medical treatment can promote endothelial wound healing or endothelial cell regeneration, there are several treatment options for your patients with clinically significant corneal endotheliopathies. In any given case, you must treat each specific endotheliopathy differently.
For example, discontinuation of contact lens wear is a conservative medical treatment for patients with contact lens-induced endotheliopathy. Or, an active medical treatment may involve continuing contact lens wear by prescribing different contact lenses with higher oxygen permeability.
In patients with Fuchs endothelial dystrophy, palliative medical treatment may involve prescribing a topical hyperosmotic solution or ointment. However, a more active approach might consist of surgical procedures, such as deep lamellar endothelial keratoplasty or penetrating keratoplasty.19
In patients with ocular inflammation, prophylactic medical treatment of a secondary endotheliopathy may involve the aggressive use of topical steroids instead of allowing the inflammation to resolve on its own.4
Regardless of the type of corneal endotheliopathy or how you elect to administer medical treatment, your ultimate goals are to prevent, reduce or eliminate corneal edema, maintain corneal transparency, and maintain the endothelial functional reserve.
Specular microscopy helps facilitate treatment by allowing you to diagnose any type of corneal endotheliopathy earlier and more accurately. Because the technology reveals information about the endothelium that is either difficult or impossible to derive from the clinical examination alone, specular microscopy helps you provide the best corneal care possible.
Dr. Thomas is in private practice in
1. Bourne WM. Biology of the corneal endothelium in health and disease. Eye 2003 Nov;17(8):912-8.
2. Sullivan-Mee. The role of ocular biomechanics in glaucoma management. Rev Optom 2008 Oct 15;145(10):49-54.
3. Edelhauser HF. The balance between corneal transparency and edemathe Proctor Lecture. Invest Ophthalmol Vis Sci 2006 May;47(5):1754-67. (All referenced photographs have been reprinted with the permission of the author.)
4. Taravella M, Walker M. Emedicine: Postoperative corneal edema. Available at: www.emedicine.com/oph/topic64.htm (Accessed May 6, 2009).
5. Philllips C, Laing R, Yee R. Specular Microscopy. In: Krachmer JH, Mannis MJ, Holland EJ (eds). Cornea, 2nd ed.
6. Liesegang TJ. Physiologic changes of the cornea with contact lens wear. CLAO J 2002 Jan;28(1):12-27.
7. McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea 2008 Jan;27(1): 1-16.
8. Corneal endothelial photography. Three-year revision.
9. Niederer RL, Perumal D, Sherwin T, McGhee CN. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol 2007 Sep;91(9):1165-9.
10. Kitagawa K,
11. Giasson CJ, Solomon LD, Polse KA. Morphometry of corneal endothelium in patients with corneal guttata. Ophthalmology 2007 Aug;114(8):1469-75.
12. Wilson SE, Bourne WM. Fuchs" dystrophy. Cornea 1988;7(1):2-18.
13. Singh D, Singh R. Emedicine: Dystrophy, Fuchs Endothelial. Available at: www.emedicine.com/oph/topic91. htm (Accessed May 6, 2009).
14. Zhang C, Bell WR, Sundin OH, et al. Immunohistochemistry and electron microscopy of early-onset Fuchs corneal dystrophy in three cases with the same L450W COL8A2 mutation. Trans Am Ophthalmol Soc 2006;104:85-97.
15. Mishima S. Clinical investigations on the corneal endothelium-XXXVIII Edward
16. Sibug ME,
17. Laing RA, Oak SS, Leibowitz HM. Specialized Microscopy of the Cornea: Specular Microscopy. In: Leibowitz HM, Waring GO (eds). Corneal Disorders: Clinical Diagnosis and Management, 2nd ed.
18. Samudre SS, Lattanzio FA, Willimas PB, Sheppard JD. Comparison of topical steroids for acute anterior uveitis. J Ocul Pharmacol Ther 2004 Dec;20(6):533-47.
19. Miller WL. Caring for bullous keratopathy. Contact Lens Spect Sept 200621(9):52.

