An 81-year-old white male was referred to our dry eye clinic for a consultation on severe keratitis sicca secondary to Sjögren’s syndrome. His ocular history was remarkable for chronic “grittiness, scratchiness and burning” in both eyes that was recalcitrant to conventional therapy.
Autologous serum eye drops (ASEDs) are not conventional therapy, but they can certainly be effective therapy for severe dry eye patients, such as those with Sjögren’s syndrome.
His current treatment regimen includes Lotemax (loteprednol etabonate 0.5%, Bausch + Lomb) b.i.d., Restasis (cyclosporine A 0.05%, Allergan) b.i.d., punctal occlusion of the lower lids, Systane Preservative-Free (Alcon) artificial tears every two to four hours while awake, and GenTeal Gel (Novartis) at bedtime, all used bilaterally. Additionally, the patient wears wraparound glasses when outdoors, and uses a cool-air mist humidifier in the living room and bedroom. His past ocular history also includes pseudophakia O.U.
His medical history is significant for high cholesterol, hypertension, primary Sjögren’s syndrome and acid reflux. Systemic medications include omeprazole, Zetia (ezetimibe, Merck), and low-dose (81mg) aspirin, all dosed once daily.
Pertinent exam findings included:
• Best-corrected visual acuity: 20/25- O.D.,
20/25- O.S.
• Mild conjunctival injection O.U.
• Corneal filaments O.U.
• Diffuse superficial punctate keratopathy O.U.
• 3+ lissamine green staining O.U.
• Well-positioned Freeman style silicone punctal plugs O.U. (lower puncta)
• Intermittent incomplete closure on blink O.U. showing mild sclera
• Adequate lid apposition O.U.
• Schirmer tear test without anesthesia: <1mm in each eye
• Blink rate: frequent (approximately every three to four seconds)
Our clinical impression was that he had severe keratitis sicca (DEWS criteria stage 3) O.U.
Given his ocular history and current clinical findings, we discussed autologous serum eye drops (ASEDs) as a treatment option.
We reviewed the risks, benefits and alternatives of autologous serum, as well as the fees involved. Upon consideration, the patient agreed to a three-month trial of ASEDs.
Accordingly, we prescribed 20% autologous serum eye drops q2h while awake O.U. We instructed him to continue Lotemax b.i.d. O.U., and GenTeal Gel at bedtime O.U., but to discontinue Restasis and Systane.
We explained that he would receive approximately 50 to 55 bottles (3ml size with a 2ml fill) of preservative-free ASEDs. We told him to store unopened bottles in the household freezer for no longer than three months. Opened bottles are to be stored in the refrigerator but must be used within 48 hours or be discarded. We told him to notify our office immediately if he notices any adverse side effects. We asked him to bring all used and partially used bottles back to our office for proper disposal.
At his one-month follow-up visit, he reported a significant improvement in his symptoms. He indicated that the burning and irritation had decreased greatly; he noted that he “couldn’t remember the last time his eyes felt this good.”
Clinically, he showed a 30% improvement in vital dye staining, and subtle improvement in tear film break-up times O.U. There was complete resolution of his corneal filaments, and visual acuity improved by one line of vision. We asked him to continue ASEDs every two hours while awake, and to use Lotemax on an as-needed basis up to twice per day.
At his three-month follow-up visit, the patient reported a mild improvement in his vision from the last visit, with only a few instances of burning and irritation that warranted the use of Lotemax. Slit-lamp biomicroscopy showed inferior staining of the cornea with sodium fluorescein, and mild staining of the conjunctiva with lissamine green. The tear prism was expectedly scant, but his blink rate had improved to approximately every six seconds.
Treatment Recommendations by Severity Level
Level 1
• Education
• Environmental/dietary modifications
• Elimination of offending systemic medications
• Artificial tear substitutes, gels/ointments
• Eyelid therapy
Level 2
If Level 1 treatments are inadequate, add:
• Anti-inflammatories
• Tetracyclines (for meibomianitis, rosacea)
• Punctal plugs
• Secretogogues
• Moisture chamber spectacles
Level 3
If Level 2 treatments are inadequate, add:
• Serum
• Contact lenses
• Permanent punctal occlusion
Level 4
If Level 3 treatments are inadequate, add:
• Systemic anti-inflammatory agents
• Surgery (lid surgery, tarsorrhaphy; mucus
membrane, salivary gland, amniotic membrane
transplantation)
Source: Wilson SE, Stulting RD. Agreement of physician treatment practices with the international task force guidelines for diagnosis and treatment of dry eye disease. Cornea. 2007 Apr;26(3):284-9.
Given the positive clinical findings and improved patient symptoms—and knowing that insurance does not cover this form of therapy—I suggested that we could try a three-month on/three-month off protocol, and see if he could maintain his comfort level with preservative-free tears during the off months.
But, with the improvement in his symptoms, the patient was adamant about continuing the ASED therapy. I agreed to prescribe another three-month supply at q2h dosing, as long as he agreed to discontinue the use of any bottles that are refrigerated for 48 hours or longer, and to bring the used and unused bottles at each subsequent follow-up visit.
Sjögren’s and DEWS
Sjögren’s syndrome (SS) is a systemic autoimmune disease in which immune cells attack and destroy host exocrine glands that produce tears and saliva.1 SS has an estimated prevalence of 0.05% to 4.8%, or about 1.3 million Americans.2 It occurs much more often in women than men, at a ratio of 9:1.2 The peak incidence is between the fourth and fifth decades of life, but it can affect all age groups and ethnic groups.
With respect to ocular involvement, the lacrimal glands are infiltrated by activated T-cell lymphocytes resulting in the release of inflammatory mediators (cytokines) into tears, promoting inflammation of the ocular surface. This inflammation adversely affects the neurosensory feedback loop vital to maintaining tear film homeostasis, eventually leading to cellular apoptosis. Patients with ocular surface disease related to Sjögren’s syndrome often show a lack of both basal and reflex tearing as well as vital dye staining that is more severe than in non-Sjögren’s dry eye patients.3,4
Based on diagnostic and treatment guidelines for stages of dry eye disease, published in the International DEWS (Dry Eye Workshop) Report, our patient presented with signs and symptoms consistent with dry eye severity level three.5 (See “ Dry Eye Severity Grading Scheme.”) One of the treatment strategies recognized by DEWS for stage three dry eye patients is autologous serum eye drops. (See “ Treatment Recommendations by Severity Level.”)
Autologous Serum
Loss of tear production can have severe visual consequences. Our natural tears are made up of key components not found in artificial tear products, such as epidermal growth factor (EGF), fibronectin and vitamin A, which support the proliferation, maturation, migration and differentiation of corneal and conjunctival epithelia.6 We also know that serum contains IgG, lysozymes and complement, which have bacteriostatic properties. If these factors are low or missing, there is increased risk of persistent epithelial defects, blurred vision, infection and scarring.
The concept of using serum as an eye drop is based on the understanding that there are biochemical similarities between an individual’s serum and natural tears.7 There have been a number of studies demonstrating the wound healing effects of ASEDs.8,9 But it has been the work of rheumatologist Robert Fox, M.D., Ph.D., at Scripps Memorial Hospital in California, and ophthalmologist Kazuo Tsubota, M.D., of Keio University School of Medicine in Tokyo, who have driven renewed interest in ASEDs for severe dry eye disease.
Dr. Fox and his colleagues were among the first to report improvement in clinical signs and symptoms in all patients treated (n=15) with 50% ASEDs for moderate to severe keratitis sicca, during a mean follow-up period of 10 months.10 Eight of the 15 patients carried the diagnosis of Sjögren’s syndrome.
1.
2.
Fifteen years later, Dr. Tsubota’s group studied the impact of 20% autologous serum on fluorescein and rose bengal staining, tear film break-up time and symptomology based on subjective face scoring in 12 patients diagnosed with Sjögren’s syndrome. Their analysis showed that within two to four weeks of treatment, there was clinically significant improvement in all objective clinical parameters as well as symptoms.11
How to Formulate ASEDs
Once a donor health questionnaire and informed consent are signed, the venipuncture site is prepped with either alcohol or povidone iodine swab. (
See figure 1.) Using an 18-gauge needle, approximately 40ml of blood is collected into six 8.5cc blood tubes. (
See figure 2.)
The collected blood is set aside to clot for two hours at room temperature. Then the blood is centrifuged at 5,600 rpm for 10 minutes. ( See figure 3.) The serum is filtered through a 25mm polyethersulfone disc filter before mixing with saline. Filtration is performed to remove fibrin strands, which are believed to lessen the effect of ASEDs. Each 8.5cc tube of blood yields approximately 4cc of serum (24cc total). ( See figure 4.)
There are no large-scale, double-blind, placebo-controlled studies that describe an optimal concentration or optimal dosing schedule for this treatment. So, in our practice, we opt for a 20% solution based on the concentration level of transforming growth factor B1 (TGF-B1) in blood serum. TGF-B1, in a dose-dependent manner, is believed to inhibit epithelial proliferation.12,13 In serum, the concentration of TGF-B1 has been found to be five times that of tears. A 20% solution keeps TGF-B1 levels in check (albeit at the expense of other key constituents, like epidermal growth factor). A controlled study comparing the safety and efficacy of 20% vs. 50% serum is certainly needed.
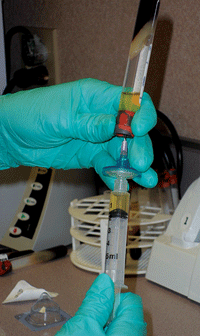
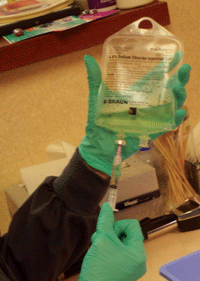
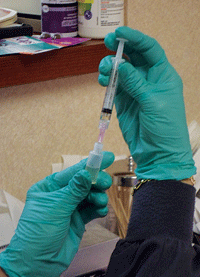
In order to obtain a 20% solution, 10cc of saline is removed from a 50cc bag; then, 10cc of 100% serum is added and mixed with the remaining 40cc of saline. So, a single blood draw produces 100cc of 20% ASEDs. (
See figure 5.) This quantity of mixed autologous serum can yield 50 sterile 3ml dropper bottles, each containing 2ml of ASEDs. (
See figure 6.) Given that 1ml equals about 20 drops, each bottle yields approximately 40 eye drops. (
See figure 7.)
Relative Risks and Complications
3,4.

The complication rate from ASEDs is relatively small.14 In studies involving 255 patients, the following complications were reported:
• Peripheral corneal infiltrate and ulcer (n=1)
• Eyelid eczema (n=2)
• Microbial keratitis in patients with an epi-defect (n=3)
• Increased discomfort or epitheliopathy (n=5)
• Temporary bacterial conjunctivitis (n=5)
• Scleral vasculitis and melting in RA patients
• Immune complex deposition with 100% serum
Bear in mind that some of these complications may have been due to the underlying disease state (i.e., uncontrolled rheumatoid arthritis) rather than a direct side effect of ASEDs.
Because ASEDs are preservative free and innately non-allergenic, there is minimal concern about toxicity from frequent dosing with the 20% formulation. However, hourly dosing of 100% serum has demonstrated immune complex deposition, so careful monitoring is still warranted with this formulation.15
When stored and used properly, the risk of serum contamination and denaturing are low. In one study of the sterility of non-preserved 20% ASEDs stored in a refrigerator, investigators found that no contamination occurred in their samples as far as three months out.16 The epitheliotrophic components in serum were also shown to remain stable for at least one month in a refrigerator and for three months in a freezer.17
Of course, with frequent dosing comes increased handling and greater variability in patient compliance regarding use and re-refrigeration. Because the risk of contamination and serum denaturing increases with lapses in refrigeration or extended single-bottle use, patients and/or caregivers must be re-educated at each visit on the importance of protocol adherence. For instance, two cases of microbial keratitis were reported when bottles of ASEDs were used for a week at a time.18 In one controlled, hospital-based study for the treatment of epithelial defects, contamination of preservative-free ASEDs did not occur until day four.19
In our practice, we dispense the serum tears in 3ml bottles and encourage a dosing regimen of every two hours while awake. This ensures that each vial will be empty within 24 to 48 hours of being refrigerated, which should prevent contamination.
While it is possible to formulate ASEDs with a preservative, preservative-free ASEDs are recommended because preservatives can negate the positive effects of the serum.20 This recommendation comes from a study that involved culturing the first and last drop of ASEDs used by 14 patients from day four to day 14. In testing a total of 134 samples, only one grew a significant ocular pathogen (S. aureus). None of the bottles grew fungi after 24 hours of use.
5-7.

Direct and Indirect Costs
In our practice, the cost for the blood draw and a three-month supply of ASEDs is $300. All patients are required to sign an Advanced Beneficiary Notice, or ABN, explaining the associated out-of-pocket expense for the drops, which are not covered by medical insurance. Assuming that the patient continues with therapy, the average annual direct cost approximates $1,200 dollars. Examination and follow-up visits are billed through insurance as usual.
While the direct cost of ASEDs may be a barrier for some patients, it is important to educate them on the cumulative indirect cost savings. As with the patient described above, many of our other patients have been able to reduce or eliminate more than one of their OTC or prescription eye drops while using ASEDs.
Patients with Sjögren’s syndrome, such as our patient described in this article, often have ocular symptoms that are recalcitrant to conventional dry eye therapies. Even in the most controlled environments, dry eye symptoms can become severe enough to adversely affect a patient’s quality of life.21 Although autologous serum is not conventional therapy, it can certainly be effective therapy for these patients.
Our patient, for instance, has had no adverse effects from the serum drops and has maintained good vision and comfort for more than a year of treatment. He is happy that our practice took the time to research the science behind the serum, and he continually reminds us of how much more comfortable his eyes have become since starting ASEDs.
When conventional therapy isn’t working for your severe dry eye patients, consider giving ASEDs a try.
 Dr. Mangan is Center Director at the Eye Center of Richmond, a multispecialty comanagement practice in Indiana and Ohio. He is chair of the refractive surgery and clinical research committees for the Eye Center of Richmond and is an adjunct clinical professor at the Indiana University School of Optometry. Ms. Lehman is a certified ophthalmic assistant and team leader at Eye Center of Richmond, as well as a certified phlebotomist.
Dr. Mangan is Center Director at the Eye Center of Richmond, a multispecialty comanagement practice in Indiana and Ohio. He is chair of the refractive surgery and clinical research committees for the Eye Center of Richmond and is an adjunct clinical professor at the Indiana University School of Optometry. Ms. Lehman is a certified ophthalmic assistant and team leader at Eye Center of Richmond, as well as a certified phlebotomist.
1. Delaleu N, Immervoll H, Cornelius J, Jonsson R. Biomarker profiles in serum and saliva of experimental Sjögren’s syndrome: associations with specific autoimmune manifestations. Arthritis Res Ther. 2008;10(1):R22.
2. Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008 Jan;58(1):15-25.
3. Pflugfelder S, Huang A, Feuer W, et al. Conjunctival cytologic features of primary Sjögren’s syndrome. Ophthalmology. 1990 Aug;97(8):985-91.
4. Tsubota K, Toda I, Yagi Y, et al. Three different types of dry eye syndrome. Cornea. 1994 May;13(3):202-9.
5. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):75-92.
6. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjögren’s syndrome. Br J Ophthalmol. 1999 Apr;83(4):390-5.
7. Quinto GG, Campos M, Behrens A. Autologous serum for ocular surface diseases. Arq Bras Oftalmol. 2008 Nov-Dec;71(6 Suppl):47-54.
8. Poon AC, Geerling G, Dart JK, et al. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001 Oct;85(10):1188-97
9. Jeng BH, Dupps WJ. Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009 Dec;28(10):1104-8.
10. Fox RI, Chan R, Michelson J, et al. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984 Apr;27(4):459-61.
11. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjögren’s syndrome. Br J Ophthalmol. 1999 Apr;83(4):390-5.
12. Imanishi J, Kamiyama K, Iguchi I, et al. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000 Jan;19(1):113-29.
13. Pancholi S, Tullo A, Khalig A, et al. The effects of growth factors and conditioned media on the proliferation of human corneal epithelial cells and keratocytes. Graefes Arch Clin Exp Ophthalmol. 1998 Jan;236(1):1-8.
14. Geerling G, MacLennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004 Nov;88(11):1467-74.
15. McDonnell PJ, Schanzlin DJ, Rao NA. Immunoglobulin deposition in the cornea after application of autologous serum. Arch Ophthalmol. 1988 Oct;106(10):1423-5.
16. Prabhasawat P, Chotikavanich S, Leelaporn A. Sterility of non-preservative eye drops. J Med Assoc Thai. 2005 Nov;88 Suppl 9:S6-10.
17. Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjögren’s syndrome. Br J Ophthalmol. 1999 Apr;83(4):390-5.
18. Poon AC, Geerling G, Dart JKG, et al. Autologous serum eye drops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. 2001 Oct;85(10):1188-97.
19. Sauer R, Blüthner K, Seitz B. Sterility of non-preserved autologous serum drops for treatment of persistent epithelial defects. Ophthalmologe. 2004 Jul;101(7):705-9.
20. Lagnado R, King AJ, Donald F, Dua HS. A protocol for low contamination risk of autologous serum drops in the management of ocular surface disorders. Br J Ophthalmol. 2004 April;88(4):464-5.
21. Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003 Jul;110(7):1412-9.