Ask any primary eye care provider about dry eye disease and they’ll likely tell you it’s an issue they encounter daily. Familiar scenarios include patients who present with “the itchy-burnies,” the “sore, chronically red eyes” or “intermittently blurred vision.” Symptoms may be dramatically worse upon awakening for some, while for others the symptoms are more intense at the end of the day. Environmental considerations, such as ambient temperature, altitude, relative humidity, air conditioning, forced-air heating and airborne pollutants, may further confound matters. Sustained visual tasks, such as reading, driving or watching television, may constitute the focal point of the patient’s complaints. Often too, the habitual or excessive use of electronic devices is consistent with more overtly symptomatic patients.
Because our definition of dry eye disease is continuously evolving, determining its precise prevalence in the general population is difficult. One recent study demonstrates a self-reported prevalence of dry eye in 14.5% of subjects.1 The disease is more common in women (17.9%) than men (10.5%), according to the report.1 Earlier studies show an estimated prevalence of 5% to 30% of the population aged 50 and older.2,3 Of course, these values are dependent upon the inclusion criteria of individual studies, so wide variability is expected. But experts do tend to agree that the prevalence of dry eye disease is on the rise, presumably due to:
Aging “baby boomers.”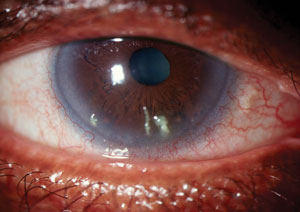
Filamentary keratitis is often seen as a complication of prolonged dry eye disease. N-acetylcysteine solution 5% to 10% used four times daily may help to alleviate this condition by providing both a mucolytic and anti-inflammatory effect. - Increased engagement with visual tasking.
- Worsening environmental conditions.
- Increased awareness of dry eye symptoms among patients.4
While the mainstay of treatment for most patients involves the use of ophthalmic lubricants—more commonly referred to as artificial tears—this therapy is often insufficient to thoroughly assuage the symptoms of moderate to severe dry eye disease. Additionally, the wide diversity of product attributes, which include such considerations as the formulation’s active ingredients (e.g., carboxymethylcellulose, hydroxypropyl methylcellulose, polyethylene glycol, polyvinyl alcohol), inactive ingredients (e.g., hydroxypropyl guar, hyaluronic acid, castor oil, edetate disodium), viscosity, osmolarity and preservatives (or lack thereof) can make for a complex management decision. Unfortunately, artificial tear selection is often more a matter of what samples are most readily available, rather than what is actually most appropriate for the patient.
Another popular management option is Restasis (0.05% cyclosporine A ophthalmic emulsion, Allergan). At present, Restasis is the only prescription medication specifically approved by the Food and Drug Administration to treat dry eye disease. While several other drug candidates have been tested and submitted over the last 12 years, none have yet been able to demonstrate the required improvement in signs and symptoms mandated by the FDA. And while Restasis has been extremely successful in terms of prescriptions written and units sold, clinicians still encounter many patients who fail to achieve complete relief with this medication. Thus, practitioners who deal with dry eye disease on a regular basis are often forced to identify alternative therapies. Many turn to medications that are FDA-approved for other conditions, but are not specifically indicated for dry eye disease. These off-label therapies commonly include topical corticosteroids (e.g., loteprednol), oral antibiotics (e.g., doxycycline) and oral cholinergic agonists (e.g., pilocarpine).5
Pharmaceutical compounding offers quite a different approach to the management of dry eye disease.
Compounding pharmacies provide much more than commercially available products. These specialized facilities actually formulate medications to the precise specifications of the physician. Individualized treatment of this type allows doctors and patients access to:6
- Alternative drug forms.
- Alternative drug concentrations.
- Alternative drug ingredients.
- Alternative drug formulations.
In other words, compounding pharmacists have the capability to produce far more diverse and patient-specific medications than the aforementioned treatment modalities for dry eye. Such pharmacists may be able to use drugs not currently available in topical form, but which are used systemically for similar pathologies. Likewise, they may formulate a higher concentration of a medication than is traditionally used for the average individual.
Compounding pharmacists can also change a formulation by adjusting the components responsible for intolerance or hypersensitivity, such as the preservative or the vehicle itself. Finally, they may be able to combine two or more active agents that are otherwise only available separately; this can enhance the efficacy of the final product while simultaneously simplifying the treatment regimen for the patient.
Let’s look at some of the more commonly compounded products that are used to manage dry eye disease today.
Anti-inflammatory Agents
The notion that dry eye disease is inflammatory in nature has been widely accepted for some time. In 2007, the Report of the International Dry Eye WorkShop stated emphatically that dry eye disease “is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface.”7 Anti-inflammatory agents used in the treatment of dry eye disease include cyclosporine, tacrolimus and nonpreserved corticosteroids.
Cyclosporine. While Restasis has been commercially available in the United States since 2003, the use of topical ophthalmic cyclosporine A dates back over 25 years. Initially employed as a steroid-sparing agent to prevent corneal transplant rejection, physicians soon realized that this compound could also help to alleviate the discomfort associated with dry eye disease.8 Researchers believe cyclosporine works by inhibiting T-cell activation and down-regulating inflammatory cytokines in the conjunctiva and lacrimal gland, thereby enhancing tear production.9,10 Additionally, this medication can increase goblet cell density while decreasing epithelial cell apoptosis.11 However, since cyclosporine is not water-soluble, early ophthalmic formulas had to be prepared using corn or peanut oil and at much higher concentrations.
Today, a number of options exist for patients who may benefit from topical cyclosporine but who have not had success with Restasis. Some of the more popular formulations include cyclosporine 0.05% in cyclodextran solution, cyclosporine 0.5% to 2% suspension in gum cellulose and cyclosporine 0.2% ophthalmic ointment. These alternative products are designed to improve tolerability through the use of different vehicles, and in some cases to enhance efficacy by using a higher concentration of the drug. Another option is cyclosporine 0.05% in combination with dexamethasone 0.01% (in cyclodextran solution). This formulation capitalizes on dexamethasone’s much faster onset of action, helping to bypass the delayed effects of cyclosporine alone. These products may be used from two to four times daily, depending upon the severity of the condition. Cyclosporine ophthalmic ointment can be used in place of Restasis for those patients who prefer it, or as an adjunctive overnight therapy to Restasis.6
Tacrolimus. This drug has a mechanism of action that is similar to cyclosporine, but its potency in vitro has been shown to be significantly greater.12,13 A calcineurin inhibitor, tacrolimus suppresses T- and B-lymphocyte activation, thereby preventing the release of inflammatory cytokines.14 Numerous studies have demonstrated the efficacy of this agent in the treatment of dry eye disease.15-17 While topical tacrolimus cream and ointment is commercially available for conditions like atopic dermatitis, the only way to obtain ophthalmic formulations of this drug is through a compounding pharmacy. Tacrolimus 0.02% eye drops represent a comparatively inexpensive option (costing roughly 40% less than 1% cyclosporine suspension) for patients who cannot tolerate or are inadequately controlled with cyclosporine. A 0.02% ointment is also available for alternative or complementary dry eye therapy. Typical dosing for ophthalmic tacrolimus is three to four times daily.
Corticosteroids. A seminal publication demonstrates that topical corticosteroid therapy was effective for patients with keratoconjunctivitis sicca whose symptoms persisted despite maximum tear replacement therapy.18 Today, corticosteroids are frequently used in the treatment of dry eye disease. Unfortunately, all of the commercially-available steroid drops (e.g., Lotemax, Vexol, FML) in the United States contain preservatives, and this can be a deterrent for patients with hypersensitivity issues. Compounding pharmacies can prepare a variety of nonpreserved topical corticosteroid preparations. Some of the more popular options include dexamethasone sodium phosphate, loteprednol etabonate, methylprednisolone sodium succinate, prednisolone sodium phosphate and prednisolone acetate. These products can be compounded at various strengths and in numerous vehicles, depending upon the need. At lower concentrations, nonpreserved steroid drops may be used for longer durations of time with less concern for complications.
 | |
| Advanced dry eye disease with ocular surface decompensation. For such presentations unresponsive to conventional therapy, options may include 0.2% cyclosporine ophthalmic ointment, 0.02% tacrolimus aqueous suspension or 1% medroxyprogesterone acetate solution. |
Hormonal compounds. There is clear and abundant evidence that androgens, estrogens and progestins play a role in autoregulation of the ocular surface and tear film.19-27 These sex hormones are all produced to a greater or lesser degree by the testes, ovaries and adrenal glands, although androgens are traditionally thought of as “male” hormones while estrogens and progestins are regarded as “female” hormones. In general, androgens such as testosterone appear to have a protective, stabilizing effect on dry eye disease by promoting secretions from both the meibomian glands and lacrimal glands.24,25 The role of estrogens and progestins is less clear, but these hormones are believed to be involved primarily in the regulation of secretory gland and ocular surface inflammation.26,27
Testosterone. Of the various hormonal compounds available for dry eye therapy, testosterone is likely the most effective and widely-publicized.
Charles Connor, PhD, OD—a professor at the Rosenberg School of Optometry, University of the Incarnate Word—developed one of the earliest formulations of topical testosterone for dry eye disease. A number of studies that use his proprietary, transdermal eyelid cream (3% to 5%) have been presented.28-33 A topical 0.5% testosterone eye drop can also be obtained via most compounding pharmacies. Testosterone supplementation appears to have its greatest impact in post-menopausal women.32,33 Research suggests that this treatment is equally suited to patients with aqueous-deficient dry eye disease and evaporative dry eye secondary to meibomian gland dysfunction.20,34
Estradiol. The most compelling argument for the role of topical estradiol in the treatment of dry eye disease comes from a randomized, controlled clinical trial published in 1998.35 Eighty-four post-menopausal women on systemic hormone replacement therapy were treated with a 0.00025% suspension of 17ß-estradiol four times daily for four months. The results of this study show improved subjective and objective dry eye measures, both of which were statistically significant when compared to baseline, and to a control group using artificial tears.35 A United States patent (also filed in 1998) suggested that solutions of 0.05% to 0.1% might be effective for treating keratoconjunctivitis sicca, even in the absence of concomitant oral estrogen therapy in post-menopausal women.36 While no commercial formulations of estradiol have been approved by the FDA, some practitioners continue to use low-concentration estradiol drops (0.01% to 0.03%) for patients with dry eye disease.
Progesterone and medroxyprogesterone. The precise role of progestins in regulating ocular surface health is not fully understood, but they are believed to have an anti-inflammatory effect, down-regulating the expression of genes associated with immune processes.37 In this capacity, they may work similarly to corticosteroids. Research with a 15% progesterone transdermal cream suggests this hormone could be useful in relieving dry eye symptoms and could potentially help a larger segment of the population than testosterone does.38 A topical solution of 0.05% progesterone combined with 0.05% testosterone is available as a dry eye therapy from many compounding pharmacies, including Leiter’s Compounding Pharmacy, one of the most well-known facilities of its type in the United States. Some pharmacies also carry 1% medroxyprogesterone acetate solution. This synthetic variant of progesterone has been advocated by some for dry eye, although it is most commonly employed as a collagenase inhibitor in conjunction with corneal surgery or trauma.39,40
Dehydroepiandrosterone. DHEA is a precursor molecule of both androgens and estrogens. It is produced by the adrenal glands and transformed in target tissues into various hormones, including testosterone and estradiol.19,41 This compound becomes especially important later in one’s life, as production of sex hormones by the testes and ovaries naturally declines.41 As with many compounded medications, there is a paucity of information in the peer-reviewed literature, but anecdotal reports suggest that 0.5% to 1% DHEA ophthalmic suspension may be of value for dry eye patients.42,43 Presumably, its conversion into testosterone, estradiol or both at the ocular level imparts the same benefits outlined previously. Like testosterone, DHEA appears to be most effective in post-menopausal women.44 Typical dosing is twice daily.42
Acetylcysteine. N-acetylcysteine (NAC) has been used medically for more than 50 years as a mucolytic agent, employed for a variety of respiratory conditions and illnesses (e.g., emphysema, cystic fibrosis).43 In the presence of accumulated mucus, NAC serves to break the disulfide bridges of high-molecular-weight glycoproteins, resulting in reduced secretion viscosity. More recent research suggests that acetyl-cysteine may also play a role in alleviating oxidative stress, which, when unchecked, stimulates an inflammatory response at the cellular level.45
Most eye care practitioners are familiar with topical NAC as a treatment option for filamentary keratitis, a condition which is often associated with severe dry eye disease.46,47 But this compound may do more than simply diminish mucus plaques; studies demonstrate a capacity for NAC to inhibit matrix metalloproteinase secretion and, at lower concentrations, facilitate wound healing in damaged corneal epithelial cells.48,49 Two recent clinical trials even showed that topical acetylcysteine may be effective for patients with meibomian gland dysfunction.50,51 Most practitioners employing NAC for ocular surface disease use solutions of 5% or 10%, four times daily. Patients should be aware that the compound may sting upon instillation, so refrigeration is recommended. The solution is also notorious for having a sulfurous or “rotten-egg” odor. Because topical NAC is prepared without a preservative, it is recommended that any unused portion be discarded after 30 days.
Autologous Serum
As we know, the initial management strategy for most forms of dry eye disease involves tear replacement therapy. Unfortunately, while artificial tears provide lubricating elements to the ocular surface, they have little inherent capacity to downregulate inflammation or facilitate healing of damaged tissues.
| Tears vs. Serum—Tale of the Tape The chemistry of blood plasma approximates that of tears to such a degree that serum, when properly diluted, provides an exceptional alternative to natural tears. The table below displays the similarities between these two vital solutions:1,2 | |||
| Tears | Serum | ||
| pH | 7.4 | 7.4 | |
| Osmolality (mOsml/kg) | 298-300 | 296 | |
| Albumin (g/100ml) | 0.392 | 4.0 - 4.8 | |
| Epidermal Growth Factor (ng/ml) | 0.2 - 3.0 | 0.5 | |
| Fibronectin (μg/ml) | 21 | 205 | |
| Globulins (g/100ml) | 0.2758 | 2.3 | |
| Lysozyme (mg/ml) | 1.4 | 6 | |
| Surface Immunoglobulin A (μg/ml) | 1190 | 2 | |
| Transforming Growth Factor Beta (ng/ml) | 2 - 10 | 6 - 33 | |
| Calcium (mmol/L) | 0.3 - 2.0 | 2.5 | |
| Potassium (mmol/L) | 26 - 42 | 4.5 | |
| Sodium (mmol/L) | 120 - 170 | 140 | |
| Water (%) | 98.2 | 91 | |
| Vitamin A (mg/ml) | 0.02 | 46 | |
| Vitamin C (mg/ml) | 0.117 | 0.02 | |
| 1. Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004 Nov;88(11):1467-74. 2. Macsai M, Mojica G. Medical management of ocular surface disease. In: Holland EJ, Mannis MJ, Lee WB, eds. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film. London: Elsevier, 2013. 271-81. | |||
Natural tears contain enzymes, growth factors, proteins and vitamins that are crucial to metabolism, and many of these elements are altered or depleted in the dry eye state. Interestingly, blood plasma contains many of the same vital elements as tears, often in similar or higher concentrations.52-55 Since the 1970s, physicians have recognized the value of blood plasma (or serum) for managing ocular surface disorders, but widespread clinical use was uncommon until about 15 years ago.55
Because this therapy is derived from the patient’s own blood and not donor tissue, the term “autologous” serum eye drops (ASED) is commonly used. ASED are patient-specific, hypoallergenic and non-preserved, and are ideally suited for individuals with hypersensitivity issues. Blood is obtained through venipuncture and separated under sterile conditions to remove the blood elements, leaving only the serum. This serum is then diluted using preservative-free demulcent solutions, usually to a 20% concentration, and bottled in small (1mL or 3mL) eye drop containers. Because ASED drops are non-preserved, the bottles must be kept frozen until the patient is ready to use them; once thawed, they may be used for up to a week, but must be kept refrigerated between doses.53 Frequency of dosing may vary, but typically the initial schedule is one drop every two hours while awake.
Although ASED provides an excellent option for patients with dry eye disease, it requires an elaborate process. Not all compounding pharmacies are equipped to formulate ASED; in fact, most require that the blood draw be performed elsewhere, such as a laboratory facility or university hospital. For this reason, many practitioners prefer to prescribe topical albumin.
Albumin. Albumin is one of the most abundant proteins found in blood plasma.56-59 Human albumin can be extracted from donated blood, purified and used for a variety of medical conditions. Typically, it is administered by infusion for the treatment of hypovolemia, hypoalbuminemia, cirrhosis, ovarian hyperstimulation syndrome and hemolytic disease of the newborn.60 In the body, albumin serves to regulate fluid distribution, bind and transport elements both crucial and harmful to metabolism (e.g., cholesterol, metal ions, fatty acids, toxins), scavenge free radicals and maintain normal capillary permeability.61
Like NAC, prepared 5% albumin solution can be formulated into eye drops with minimal effort using aseptic techniques. Several prospective trials using both animal and human models have demonstrated beneficial effects of albumin drops when used to treat severe dry eye and persistent corneal epithelial defects.56-59 This 5% solution can be stored in the refrigerator (at ~44° F) for up to four weeks, but any unused portion should be discarded after that.
Despite the frequency with which we encounter dry eye disease and the seemingly vast array of products designed to manage it, patients with advanced presentations can quickly exhaust these options. For such individuals, “outside-the-box” thinking is often required. Pharmaceutical compounding offers an abundance of medical therapies that may alleviate discomfort and promote ocular surface healing when conventional methods fail to deliver.
Disclosure: Dr. Kabat is a consultant for Bio-Tissue and serves in an advisory capacity for BlephEx, Ocusoft and TearScience. Additionally, he is a speaker for Shire and a clinical investigator for Thermi. Dr. Kabat has no financial interest in any of the products mentioned in this article.
1. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014 Apr;157(4):799-806.2. The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):93-107.
3. Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000 Sep;118(9):1264-8.
4. Lin MC, Polse KA. Improving care for patients with dry eye symptoms: See What the Experts Say. Optom Vis Sci. 2015 Jul 13. [Epub ahead of print].
5. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):163-78.
6. Autry JC. Mix it up: When to call a compounding pharmacist. Review of Optometry. 2012 Jul; 149(7):30-7.
7. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007 Apr;5(2):75-92.
8. Donnenfeld ED, Perry HD. Experience expands the reach of restasis. Review of Ophthalmology. 2003 Aug;10(8):54-6.
9. Laibovitz RA, Solch S, Andriano K, et al. Pilot trial of cyclosporine 1% ophthalmic ointment in the treatment of keratoconjunctivitis sicca. Cornea. 1993 Jul;12(4):315-23.
10. Stern ME, Gao J, Siemasko KF, at al. The role of the lacrimal gland functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004 Mar;78(3):409-16.
11. Sall K, Stevenson OD, Mundorf T, Reis B. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000 Apr;107(4):631-9.
12. Hessen M, Akpek E. Dry eye: an inflammatory ocular disease. J Ophthal Vis Res. 2014 Apr;9(2):240-50.
13. Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from Streptomyces. I. Fermentation isolation, and physio-chemical and biological characteristics. J Antibiot (Tokyo). 1987 Sep;40(9):1249-55.
14. Fujita E, Teramura Y, Shiraga T, et al. Pharmacokinetics and tissue distribution of tacrolimus (FK506) after a single or repeated ocular instillation in rabbits. J Ocul Pharmacol Ther. 2008 Jun;24(3):309-19.
15. Sanz-Marco E, Udaondo P, Garcia-Delpech S, et al. Treatment of refractory dry eye associated with graft versus host disease with 0.03% tacrolimus eyedrops. J Ocul Pharm Ther. 2013 Oct;29(8):776-83.
16. Moscovici BK, Holzchuh R, Chiacchio BB, et al. Clinical treatment of dry eye using 0.03% tacrolimus eye drops. Cornea. 2012 Aug;31(8):945-9.
17. Moscovici BK, Holzchuh R, Sakassegawa-Naves FE, et al. Treatment of Sjogren’s syndrome dry eye using 0.03% tacrolimus eye drop: Prospective double-blind randomized study. Cont Lens Anterior Eye. 2015 May 5. [Epub ahead of print].
18. Marsh P, Pflugfelder SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology. 1999 Apr;106(4):811-6.
19. Truong S, Cole N, Stapleton F, Golebiowski B. Sex hormones and the dry eye. Clin Exp Optom. 2014 Jul;97(7):324-36.
20. Sullivan DA, Yamagami H, Liu M, et al. Sex steroids, the meibomian gland and evaporative dry eye. Adv Exp Med Biol. 2002;506(Pt A):389-99.
21. Suzuki T, Schaumberg DA, Sullivan BD, et al. Do estrogen and progesterone play a role in the dry eye of Sjögren’s syndrome? Ann N Y Acad Sci 2002 Jun;966:223-5.
22. Pflugfelder SC. Hormonal deficiencies and dry eye. Arch Ophthalmol. 2004 Feb;122(2):273-4.
23. Wickham LA, Rocha EM, Gao J, et al. Identification and hormonal control of sex steroid receptors in the eye. Adv Exp Med Biol 1998;438:95-100.
24. Yamagami H, Schirra F, Liu M, et al. Androgen influence on gene expression in the meibomian gland. Adv Exp Med Biol. 2002;506(Pt A):477-81.
25. Sullivan D, Block L, Pena J. Influence of androgens and pituitary hormones on the structural profile and secretory activity of the lacrimal gland. Acta Ophthalmol Scand. 1996 Oct;74(5):421-35.
26. Mostafa S, Seamon V, Azzarolo AM. Influence of sex hormones and genetic predisposition in Sjögren’s syndrome: A new clue to the immunopathogenesis of dry eye disease. Exp Eye Res. 2012 Mar;96(1):88-97.
27. Suzuki T, Sullivan DA. Estrogen stimulation of proinflammatory cytokine and matrix metalloproteinase gene expression in human corneal epithelial cells. Cornea. 2005 Nov;24(8):1004-9.
28. Connor CG. Transdermal testosterone delivery for the treatment of dry eye. Poster presented at the American Academy of Optometry meeting, Orlando, FL. December 12-15, 2002.
29. Connor CG. Symptomatic relief of dry eye assessed with the OSDI in patients using 5% testosterone cream. Invest Ophthalmol Vis Sci. 2005; 46(13):2032.
30. Connor CG, Jones M. Long term observation of patients using transdermal testosterone cream for the treatment of dry eye. Poster presented at the American Academy of Optometry meeting, San Diego, CA. December 8-11, 2005.
31. Connor CG. Evaporative dry eye treated with transdermal testosterone. Invest Ophthalmol Vis Sci. 2008; 49(13):120.
32. Connor CG. Androgenic cream treatment most efficacious for women 40 to 60 with dry eye. Invest Ophthalmol Vis Sci. 2009;50(13):46-56.
33. Connor CG. Testosterone cream increases contact lens wear time in menopausal dry eye patients. Invest Ophthalmol Vis Sci. 2010;51(13):62-71.
34. Schiffman RM, Bradford R, Bunnell B, et al. A multi-center, double-masked, randomized, vehicle-controlled, parallel group study to evaluate the safety and efficacy of testosterone ophthalmic solution in patients with meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2006;47(13):5608.
35. Sator MO, Joura EA, Golaszewski T, et al. Treatment of menopausal keratoconjunctivitis sicca with topical oestradiol. Br J Obstet Gynaecol. 1998 Jan;105(1):100-2.
36. Lubkin, V. Drugs for topical application of sex steroids in the treatment of dry eye syndrome, and methods of preparation and application. Virginia Lubkin, assignee. Patent US6096733 A. 10 Dec. 1998. Print.
37. Suzuki T, Schirra F, Richards SM, et al. Estrogen and progesterone control of gene expression in the mouse meibomian gland. Invest Ophthalmol Vis Sci. 2008 May;49(5):1797-808. 33.
38. Connor CG. The use of progesterone cream to treat dry eye. Invest Ophthalmol Vis Sci. 2007;48(13):378.
39. Mazzotta C, Balestrazzi A, Tarantello A, et al. The effects of medroxyprogesterone in corneal melting after Boston type I keratoprosthesis implantation. Minerva Oftalmologica. 2012 Dec;54(4):159-63.
40. Foster CS. Connective Tissue / Collagen Vascular Diseases. In: Smolin G, Foster CS, Azar DT, Dohlman CH, eds. Smolin and Thoft’s The Cornea: Scientific Foundations and Clinical Practice, 4th Edition. Philadelphia: Lippincott Williams & Wilkins, 2005. 515-50.
41. Samaras N, Papadopoulou MA, Samaras D, Ongaro F. Off-label use of hormones as an antiaging strategy: a review. Clin Interv Aging. 2014 Jul 23;9:1175-86.
42. Giannoni AG. Bring Tears to Their Eyes. Optometric Management. 2012 Nov;47(11):24-31.
43. Reed K. Dry Eye Treatment: The Unusual Suspects. Review of Cornea & Contact Lenses. 2013 Mar;150(2):24-6.
44. Connor CG, Fender J. Comparison of androgenic supplemented artificial tears. Invest Ophthalmol Vis Sci. 2002;43(13):66.
45. Aitio ML. N-acetylcysteine -- passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006 Jan;61(1):5-15.
46. Absolon MJ, Brown CA. Acetylcysteine in kerato-conjunctivitis sicca. Br J Ophthalmol. 1968 Apr;52(4):310-6.
47. Albietz J, Sanfilippo P, Troutbeck R, Lenton LM. Management of filamentary keratitis associated with aqueous-deficient dry eye. Optom Vis Sci. 2003 Jun;80(6):420-30.
48. Ramaesh T, Ramaesh K, Riley SC, et al. Effects of N-acetylcysteine on matrix metalloproteinase-9 secretion and cell migration of human corneal epithelial cells. Eye (Lond). 2012 Aug;26(8):1138-44.
49. Aldavood SJ, Behyar R, Sarchahi AA, et al. Effect of acetylcysteine on experimental corneal wounds in dogs. Ophthalmic Res. 2003 Nov-Dec;35(6):319-23.
50. Akyol-Salman I, Azizi S, Mumcu U, Baykal O. Efficacy of topical N-acetylcysteine in the treatment of meibomian gland dysfunction. J Ocul Pharmacol Ther. 2010 Aug;26(4):329-33.
51. Akyol-Salman I, Azizi S, Mumcu UY, Ateş O, Baykal O. Comparison of the efficacy of topical N-acetyl-cysteine and a topical steroid-antibiotic combination therapy in the treatment of meibomian gland dysfunction. J Ocul Pharmacol Ther. 2012 Feb;28(1):49-52.
52. Quinto GG, Campos M, Behrens A. Autologous serum for ocular surface diseases. Arq Bras Oftalmol. 2008 Nov-Dec;71(6 Suppl):47-54.
53. Geerling G, Maclennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol. 2004 Nov;88(11):1467-74.
54. Pan Q, Angelina A, Zambrano A, et al. Autologous serum eye drops for dry eye. Cochrane Database Syst Rev. 2013 Aug 27;8:CD009327.
55. Azari AA, Rapuano CJ. Autologous serum eye drops for the treatment of ocular surface disease. Eye Contact Lens. 2015 May;41(3):133-40.
56. Schargus M, Kohlhaas M, Unterlauft JD. Treatment of severe ocular surface disorders with albumin eye drops. J Ocul Pharmacol Ther. 2015 Jun;31(5):291-5.
57. Higuchi A, Ueno R, Shimmura S, et al. Albumin rescues ocular epithelial cells from cell death in dry eye. Curr Eye Res. 2007 Feb;32(2):83-8.
58. Unterlauft JD, Kohlhaas M, Hofbauer I, et al. Albumin eye drops for treatment of ocular surface diseases. Ophthalmologe. 2009 Oct;106(10):932-7.
59. Shimmura S, Ueno R, Matsumoto Y, et al. Albumin as a tear supplement in the treatment of severe dry eye. Br J Ophthalmol. 2003 Oct;87(10):1279-83.
60. Boldt J. Use of albumin: an update. Br J Anaesth. 2010 Mar;104(3):276-84.
61. Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood Transfus. 2013 Sep;11 Suppl 4:s18-25.

