 A 72-year-old white female with pseudoexfoliation syndrome (PXS) in her right eye converted to pseudoexfoliative glaucoma (PXG) in mid-2007, indicated by increasing intraocular pressure and subtle changes to her neuroretinal rim.
A 72-year-old white female with pseudoexfoliation syndrome (PXS) in her right eye converted to pseudoexfoliative glaucoma (PXG) in mid-2007, indicated by increasing intraocular pressure and subtle changes to her neuroretinal rim.
Diagnostic Data
When initially seen in 2005, this patient’s IOP measured 21mm Hg O.D. and 12mm Hg O.S. Her anterior segment in the right eye was characterized by classic findings of PXS, including pseudoexfoliative material noted on the anterior lens capsule, the papillary ruff and in the anterior chamber angle.
Optic nerve cup-to-disc ratios were 0.55 x 0.55 O.D. and 0.50 x 0.55 O.S., with healthy, plush, well-perfused neuroretinal rims. Threshold visual fields (using standard automated perimetry) were normal O.U. She reported no family history of glaucoma.
Because of the PXS, I scheduled her to return for follow-up every six months.
But in 2006, her IOP in her right eye began to fluctuate, with pressures ranging as high as 31mm Hg. I adjusted the patient’s follow-up visits to every four months.
When seen in June 2007, her best-corrected visual acuity was 20/20 O.D. and O.S. through minimally hyperopic astigmatic correction. Pupils were equal, round and reactive to light and accommodation, with no afferent defect. IOP measured 28mm Hg O.D. and 17mm Hg O.S. Central corneal thickness measured 549μm O.D. and 537μm O.S. Threshold visual fields (SAP) were normal O.U. Gonioscopy demonstrated 4+ open angles O.U. with minimal trabecular pigmentation and normal iris root configurations.
Stereoscopic disc evaluation indicated cup-to-disc ratios of 0.55 x 0.60 O.D. and 0.55 x 0.55 O.S. I took stereo optic nerve photos; overlay analysis using similar images (obtained in 2004) revealed a subtle change in the vertical component of the neuroretinal rim of the right eye.
Diagnosis and Management
When she returned in September 2007, we performed retinal topography (Heidelberg Retinal Tomograph III). The Topographic Change Analysis confirmed loss of neuroretinal rim tissue in the right eye. Other studies during this visit were unchanged from previous visits. Accordingly, I prescribed 1gtt Lumigan (bimatoprost, Allergan) h.s. O.D.
Subsequent follow-up visits have demonstrated stabilization of the neuroretinal rim loss, no SAP field loss, and post-treatment IOP in the right eye ranging from 15mm Hg to 19mm Hg. The IOP in the left eye has remained unchanged since baseline examination.
Meanwhile, she developed mild nuclear cataracts with cortical spokes in both eyes, and a subsequent reduction of best-corrected visual acuity to 20/30- O.D. and O.S. Her macular and retinal vascular evaluations have remained stable, characterized by fine retinal pigment epithelium granulation and minimal arteriolarsclerotic retinopathy. Her systemic medications at her most recent visit in March 2012 included lisinopril, metoprolol, Lipitor (atorvastatin, Pfizer) and low-dose (81mg) aspirin.
At the conclusion of this most recent visit—after reassuring her that all was stable and that she needed to continue taking her glaucoma drops—she looked me squarely in the eye and asked, “Doc, how did I get this?”
Discussion
In a previous column, I discussed pseudoexfoliation syndrome and pseudoexfoliative glaucoma (“
What’s Your Next Step?” March 2011). We know that a relatively high number of patients with PXS go on to develop PXG.1 Studies vary on the percentage of conversion from PXS to PXG—some studies estimate that as many as 50% of PXS patients ultimately convert to PXG. But, a more realistic percentage is likely about one third.2
In addition, PXS and PXG have racial and genetic predispositions; the LOXL1 gene mutation recently has been linked to the development of exfoliation.3
Furthermore, research indicates that pseudoexfoliation is not a disease specific to the eye; rather it appears to be a systemic disease affecting basement membranes, with its most visible signs found in the eye. We also know that patients with PXG generally do well when undergoing laser trabeculoplasty to control IOP.
But the patient’s question was interesting. We’ve all seen glaucoma patients who have asked us “How did I get this?” as if it was something they did that brought the condition on. Our usual answer is that, other than genetics, steroid use and anatomical issues, glaucoma is something that develops separate from anything that a person does (though there are indications that inversion, caffeine intake, tight collars, etc., can affect IOP in the short term).
But, some recent studies of PXS and PXG indicate that maybe something that the patient actually inadvertently does affects the incidence of both diseases.
For instance, researchers at the Kellogg Eye Center performed a retrospective study of 3,367 individuals diagnosed with pseudoexfoliation syndrome to see if there is a link between where they lived and the incidence of PXS.4 Interestingly, by looking at geographic and climate data from the individuals identified with PXS, the researchers found that patients who lived in the upper tier of the continental United States had the highest incidence of exfoliative syndrome. Meanwhile, those in the southernmost tier had the lowest incidence of the syndrome, and those in the middle tier had an intermediate incidence.
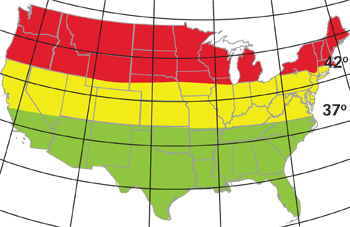
In the United States, people who live in the northern tier (above 42° latitude) have the highest incidence of exfoliative syndrome. Those in the southern tier (below 37°) have the lowest incidence.
When the data was stratified by temperature, higher average temperatures measured in January and July were associated with a significantly lower risk of exfoliation.4 But, for each additional sunny day, the risk increased by 1.5%. Furthermore, these findings did not change when white participants were removed from the study.
A more recent prospective study of 348 suspects or individuals diagnosed with exfoliative glaucoma (out of a pool of more than 120,000 health care professionals) looked at the relationship between exfoliative glaucoma and demographic and geographic data.5 This study found that female gender and increased age are associated with a higher incidence of PXG, and that ancestry, including Scandinavian descent, is not.
This study also corroborated that individuals who live further south in the U.S. are less likely to develop PXG than those living in the northernmost states. As it turned out, this patient had moved to North Carolina in 1994, but had been born in Pennsylvania and lived there for most of her life.
While these studies show a link between where one lives in the U.S. and exfoliative syndrome and glaucoma (independent of race and ethnicity), other known factors are also involved. But, they do beg the question about how environmental issues play a role in its genesis.
So, how did this patient “get it,” to use her words? Maybe, just maybe, it had something to do with where she lived during her life (especially the early years), among other things.
1. Mitchell P, Wang JJ, Hourihan F. The relationship between glaucoma and pseudoexfoliation: the Blue Mountains Eye Study. Arch Ophthalmol. 1999 Oct;117(10):1319-24.
2. Shazly TA, Farrag AN, Kamel A, Al-Hussaini AK. Prevalence of pseudoexfoliation syndrome and pseudoexfoliation glaucoma in Upper Egypt. BMC Ophthalmol. 2011 Jun 27;11:18.
3. Khan TT, Li G, Navarro ID, et al. LOXL1 expression in lens capsule tissue specimens from individuals with pseudoexfoliation syndrome and glaucoma. Mol Vis. 2010 Nov 2;16:2236-41.
4. Stein JD, Pasquale LR, Talwar N, et al. Geographic and climatic factors associated with exfoliation syndrome. Arch Ophthalmol. 2011 Aug;129(8):1053-60.
5. Kang JH, Loomis S, Wiggs JL, et al. Demographic and geographic features of exfoliation glaucoma in 2 United States-based prospective cohorts. Ophthalmology. 2012 Jan;119(1):27-35.

