A Japanese woman in her 70s has become the world’s first patient to receive tissue derived from induced pluripotent stem cells as part of an experimental treatment to repair damage caused by age-related macular degeneration.
 | |
|
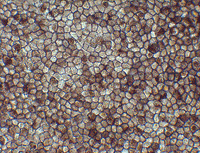
Retinal pigment epithelium (RPE) cells derived from human induced pluripotent stem cells.
|
During the two-hour procedure at the Institute for Biomedical Research and Innovation in Kobe, Japan, surgeons grafted a single 1.3mm x 3.0mm sheet of retinal pigment epithelium cells into the subretinal space of one eye. No serious hemorrhaging or complications occurred.1
The epithelium sheet was developed by ophthalmologist Masayo Takahashi, MD, PhD, of the RIKEN Center for Developmental Biology using pluripotent stem cells, which have the unique potential to differentiate into almost any type of cell found within the body. The most prominent type of pluripotent stem cell is the embryonic stem cell; however, limited resources and ongoing controversy has prevented widespread adoption. Induced pluripotent stem cells, on the other hand, are artificially derived from an adult somatic cell. In this case, the pluripotent stem cells were derived from the patient’s own skin cells, then converted into retinal pigment epithelium cells and grown into the RPE cell sheet.
This pilot study follows an earlier preclinical safety and feasibility evaluation of human-induced pluripotent RPE cell sheets created without using artificial scaffolds.2 The research examined cell morphology, physiological behavior, gene expression, immunogenicity and tumor formation in rodent and non-human primate models.
 |
|
| RPE cells formed into a sheet to be implanted into the subretinal space of a patient with AMD. Image: RIKEN Center for Developmental Biology. |
Researchers believe the use of pluripotent stem cells will improve upon current treatments that are designed to halt neovascularization, but do not repair photoreceptor cell damage that may have already occurred prior to administration. The RPE graft, however, could stop further damage and may even eventually stimulate some healing of the epithelium.
Additionally, harvesting the patient’s own cells from an innocuous place such as the skin reduces potential complications associated with immune rejection and avoids invasive harvesting procedures.
The current patient will be monitored for both functional integration and adverse reactions during a one-year initial intensive observation period, with subsequent follow-up observation for an additional three years. Five additional patients will be treated using the same procedure as part of the pilot study.
|
‘Gatekeeper Cells’ are Stiffer in Eyes with Glaucoma Endothelial cells in Schlemm’s canal are stiffer in glaucomatous eyes than in healthy eyes, likely accounting for increased outflow resistance, according to a new study. This stiffness limits the cells’ ability to deform and allow aqueous humor to cross the endothelium and drain into Schlemm’s canal. “Rather than being seen as a simple mechanical barrier to filtration, the endothelium of [Schlemm’s canal] is seen instead as a dynamic material whose response to mechanical strain leads to pore formation and thereby modulates the resistance to aqueous humor outflow. In the glaucomatous eye, this process becomes impaired,” the authors conclude. “Our work shows that cells of this endothelial layer act as mechanical gates,” says lead author Mark Johnson, PhD, professor of biomedical engineering at Northwestern University. “Therapeutic strategies that alter the stiffness of these cells potentially could lead to a cure for this debilitating disease.” Overby DR, Zhou EH, Vargas-Pinto R, et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc Natl Acad Sci USA. 2014 Sep 23;111(38):13876-81. |
1. RIKEN Center for Developmental Biology. First RPE cell sheet graft transplant. Available at: www.riken.jp/en/pr/topics/2014/20140912_1/. Accessed October 6, 2014.
2. Kamao H, Mandai M, Okamoto S, et al. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2014 Jan 23;2(2):205-18.

