Some research has suggested an overlap or a link, or even that such diseases stem from the same source. Indeed, glaucoma’s status as a neurodegenerative disorder may place it alongside these devastating conditions. The silver lining is that their shared features may result in an inclusive screening protocol that extends beyond examination of the optic nerve.
Of course, the biological basis of glaucoma has many contributing factors, some of which are still poorly or incompletely understood.2 However, newer research indicates that glaucoma may originate in the brain. Studies on glaucoma-induced mice have shown a type of distal dieback of retinal ganglion cell (RGC) axons that begins as far back as the superior colliculus in the mid-brain.3-6 This dieback is preceded by damaged axoplasmic transport that progresses distally to proximal to the optic nerve, implying a direct link to the central nervous system (CNS).3-6 This is but one scrap of evidence that glaucoma may be more of a brain disorder than an ocular disease.
|
|
|
|
|
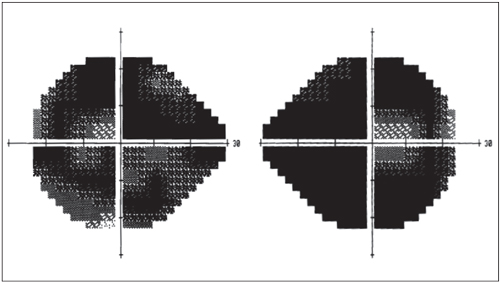
A severe end-stage glaucoma patient shows minimal residual visual field patency. Recent research by Sponsel et al. theorizes that late patency is under CNS control.8 |
In this article, we’ll discuss the available research, and how it may change the way that we evaluate glaucoma—and perhaps compel us to identify neurodegenerative disorders further along the visual pathway.
CNS and Glaucoma IMAGE
The eyes are an extension of the brain. But you wouldn’t know it by looking at visual fields. Bilateral visual field loss in glaucoma is only orderly (respecting the vertical midline) in retrochiasmal pathologies. Otherwise, glaucoma defects are typically asymmetric, with loss occurring erratically. The orderly retrochiasmal defects and the apparent disorder of glaucoma defects appears to preclude glaucomatous disease from having a direct connection with the entirety of the brain.
However, Gupta et al. reported the first case of human glaucoma demonstrating neurodegenerative changes in the brain, which correlated with clinical, optic nerve head and visual field findings. This evidence suggests that glaucoma involves the intracranial optic nerve, lateral geniculate nucleus and visual cortex.7
Based on that research, Sponsel et al. recently theorized that binocular visual field patency in late glaucoma is under CNS control.8 The team reasoned that CNS involvement could be confirmed if complementary islands of vision could be preserved in a pair of eyes with severe disease.9 In their study, the authors paired each glaucoma patient’s left visual field locus with its
|
Sleep Apnea, Glaucoma and the Nervous System
Investigators also considered the role CSF pressure might play in RNFL loss for patients with obstructive sleep apnea (OSA), one of the most common sleep disorders.19
|
directly corresponding right locus.8 This pairing resulted in a non-random sparing of the loci corresponding to the defect in the opposite eye. The result was analogous to a jigsaw puzzle fitting together as the corresponding fields matched. This “jigsaw” phenomenon is highly suggestive of focal neurodegeneration coordinated by the CNS.8 Essentially, islands of monocular function are integrated together in a process that maximizes the binocular visual field.9 The result appears to confirm meticulous order to a process that was previously considered random and unorderly.8
Additional evidence suggests glaucomatous damage extends along the entire visual pathway, not just RGC axons.9 Frezzotti et al. conducted an MRI study comparing a normal control group to a group of patients with advanced primary open angle glaucoma (POAG) who had no additional neurological or ophthalmological disorders.9 The authors concluded that the findings of widespread brain abnormalities in POAG (such as alterations of the integrity of the white matter and alterations in the volume of the grey matter) extended beyond the visual system in these patients.9 In addition to structural alterations, the authors found functional connectivity changes among the resting brain networks—specifically the visual and working memory networks. These results may explain why advanced POAG patients may suffer from the impairment of object identification.
|
|
|
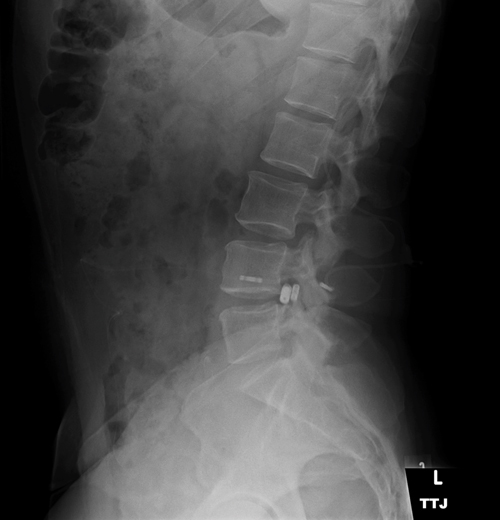
A pseudotumor cerebri patient with intrathecal spinal catheter, used to determine lumbar peritoneal shunt function by continuous monitoring of CSF pressure. Increased cerebrospinal fluid pressure has been shown to reduce the translaminar pressure gradient, acting in a protective manner.
|
|
CSF Pressure and Glaucoma
Research also indicates a link between low cerebrospinal fluid pressure and the development of glaucoma.
Anatomic investigations have shown that a translaminar (across the lamina cribrosa) pressure gradient is formed when intraocular pressure (IOP) is resisted by a combination of retrolaminar tissue pressure and orbital cerebrospinal fluid (CSF) pressure.10 This translaminar pressure difference may play a role in the pathogenesis of optic nerve disease, including glaucoma.11 Low CSF pressure, leading to a high translaminar pressure gradient, may to result in barotrauma and subsequent damage to the lamina.11
Conversely, an increased CSF pressure has been shown to be protective in ocular hypertension patients. Here, as the overall translaminar pressure gradient is balanced and, in effect, decreased compared with what it would have been if the CSF pressure was lower, the lamina does not pathologically bow backward to induce classic glaucomatous loss.11
Past clinical studies have shown lower lumbar CSF pressure measurements in patients with normal tension glaucoma vs. patients with high tension glaucoma.13,14
A recent primate study further enforces the notion that low CSF pressure alone might contribute toward retinal ganglion cell injury and loss. Yang et al. subjected rhesus monkeys to implantation of a lumbar-peritoneal cerebrospinal fluid shunt. In the study, the shunt group had the CSF pressure lowered to 40mm H2
0, while the shunt remained closed in the control group. Fifty percent of the study monkeys developed morphological disc changes, including progressive reduction in retinal nerve fiber layer thickness, reduction in neuroretinal rim area and volume, and increased cup-to-disc ratio. No morphological changes developed in the control group eyes.17
If these findings are applicable to humans, low cerebrospinal fluid pressure may be a risk factor for all forms of optic neuropathy, including glaucoma.13-17
The population-based Beijing Eye Study of 3,468 individuals further supports the possible role of low CSF pressure in the pathogenesis of human OAG. It shows that an estimated trans-lamina cribrosa pressure difference has a better association with glaucoma presence and extent of glaucomatous optic neuropathy than IOP.18
Alzheimer’s Disease and Glaucoma
Recent studies associate glaucoma with Alzheimer’s disease (AD), drawing comparisons between the two as they share many mechanistic and biological similarities.23
AD and glaucoma are both chronic neurodegenerative conditions occurring with higher incidence in patients with advanced age. Both diseases present as a continuum, with greater uncertainty of diagnosis earlier rather than later in the disease process. However, the relationship between the two diorders remains obscure.
Alzheimer’s disease is a progressive neurodegenerative disorder characterized by cognitive and memory deterioration, as well as changes in personality, behavioral disturbances and an impaired ability to perform activities of daily living.24 It is the most common form of dementia in the elderly, accounting for an estimated 60% to 80% of cases.25 The current diagnosis relies on neuropathology testing, which is both costly and invasive. Correlation between neuropathology and clinical features may be poor because the signs and symptoms can vary with the extent of brain location and involvement.26,27
| |
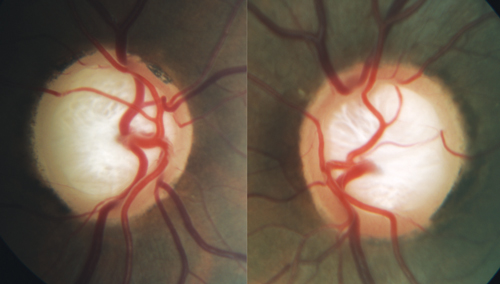
| A moderate stage glaucoma patient with substantial loss and reconfiguration of laminar tissue. Anatomic investigations have shown that a translaminar pressure gradient is formed when IOP is resisted by a combination of retrolaminar tissue pressure and orbital cerebrospinal fluid pressure. |
Brains of Alzheimer’s patients are characterized by the presence of extracellular senile plaques containing the protein amyloid-β and intracellular neurofibrillary tangles (NFTs) of abnormally phosphorylated protein tau.24,28 The accumulation of amyloid-β is believed to interfere with the neuron-to-neuron communication at synapses, and contribute to cell death.25 This neurotoxic damage and neuronal death occurs across the brain, including the visual cortex.26 Neurotransmitter function is also impaired, particularly levels of acetylcholine, known to be crucial to the functioning of retinal cells.30,31
The axons of the RGCs create the nerve fiber layer, the optic nerve and then synapse directly with multiple regions of the brain.26-34 Hinton and associates, who in 1986 first reported histopathologic evidence of retinal degeneration in AD patients,32 conducted a postmortem analysis on the optic nerves and retinas of patients with and without AD. The AD samples demonstrated widespread axonal degeneration in the optic nerves. This degeneration was accompanied by substantially reduced RNFL thickness and significant loss of RGCs.32 Large diameter RGCs (magnocellular, or M, cells) and axons were predominantly affected.33 Aggregates of amyloid-β and neurofibrillary plaques of tau were found in the retina of AD patients.26
Retinal neurons are now known to be sensitive to the neurotoxity of amyloid-β aggregates.30 Further, research associates amyloid-β deposition in the RGC layer with RGC apoptosis.26-34 This also accounts for, and contributes to, thinning of the RNFL along with the morphological changes characteristically seen in optic nerve heads of known glaucoma patients.
Several studies using multiple imaging technologies have examined the relationship between RNFL and AD.36-39 As RGC apoptosis is associated with proteins found in AD, the RNFL can serve as a biomarker for AD. Noninvasive in vivo measurements of that biomarker could lead to easier AD diagnosis.26-36
|
Can SD-OCT Detect Alzheimer’s Disease?
The results, using spectral-domain optical coherence tomography (SD-OCT), demonstrated a significant reduction in the total RNFL thickness in the AD group vs. healthy controls.35 Overall RNFL thickness was most influenced by selective thinning of the superior quadrant.35 The other three quadrants were similar to the healthy controls.35 This selective thinning of the superior quadrant was found in previous studies using time-domain OCT.36-39 Superior RNFL thinning is consistent with the classic inferior visual field defects seen in patients with both AD and OAG. To explain this relationship, investigators cite primary involvement of axons from the superior retina in the cuneal gyrus of the primary visual cortex.35 The authors propose, however, the cause of RNFL thinning in AD is likely due to the death of retinal ganglion cells coupled with retrograde degeneration from loss of cortical neurons.35 They conclude the use of SD-OCT is rapid, repeatable, noninvasive and could operate as a form of early detection for AD.35 The study also seems to imply doctors consider the presence of AD in normal-tension glaucoma cases.35 |
|
This information implies a positive association between glaucoma and AD.26-42 If there were a positive association, patients diagnosed with one condition might be automatically screened for the other condition, streamlining the process.
However, not all research shows a link between AD and glaucoma. Keenan, et al. found no additional risk of AD following diagnosis of POAG.43 The authors concluded that, although POAG and AD are neurodegenerative diseases that share some pathological features, their coexistence at the individual level is no greater than chance.43 A study from Denmark investigating the 25-year rate of developing dementia/Alzheimer’s disease in patients previously diagnosed with normal-tension glaucoma found no association between the two diseases.44
Ou et al. also concluded no positive association between the two diseases. In fact, the OAG patients were actually found to have a decreased rate of AD diagnoses.45
In contrast to the above results, a longitudinal record linkage study randomly sampled a data set of two million beneficiaries from the Taiwan National Health Insurance Research database who were followed for eight years.46 Investigators concluded that in elderly patients, POAG is a significant predictor of AD, but not of Parkinson’s disease.
The first prospective longitudinal study evaluating the relationship between OAG and dementia, with active screening for both dementia and glaucoma, found a strong association between OAG and dementia.50 Although their analysis did not detail the etiology of dementia, the authors inferred a link between AD and glaucoma given that AD was represented as the cause of dementia for most of the patients.44
All in all, the multitude of biological and mechanistic similarities between these two neurodegenerative disorders is vast. But, to date, no consensus on the association exists in the literature.27-47
Glaucoma’s complexity is astounding, and the evidence for co-contributing CNS disease is truly compelling. Although these initial reports are critical in furthering our understanding of the disease, more investigation is needed before widespread adoption of new evaluation and treatment strategies.
The future possibility of unlocking the seemingly intangible mysteries of the brain and glaucoma becomes more tangible with each additional scientific report. At present we must hone our skill in early diagnosis and management of this disease with technology and knowledge that is clinically applicable today.
1. American Optometric Association. Optometric Clinical Practice Guideline: Care of the Patient with Open Angle Glaucoma. Available at: http://www.aoa.org/documents/optometrists/CPG-9.pdf (accessed December 14, 2014).2. Weinreb RN, Aung T, Medeiros FA. The Pathophysiology and Treatment of Glaucoma A Review JAMA. 2014;311(18):1901-11
3. Crish SD, Sappington RM, Inman DM, et al. Distal axonopathy with structural persistence in glaucomatous neurodegeneration. Proc Natl Acad Sci U S A. 2010;107:5196–5201.
4. Schlamp CL, Li Y, Dietz JA, et al. Progressive ganglion cell loss and optic nerve degeneration in DBA/2J mice is variable and asymmetric. BMC Neurosci. 2006;7:66.
5. Crish SD, Dapper JD, MacNamee SE, et al. Failure of axonal transport induces a spatially coincident increase in astrocyte BDNF prior to synapse loss in a central target. Neuroscience. 2013;229:55–70.
6. Zhang S, Wang H, Lu Q, et al. Detection of early neuron degeneration and accompanying glial responses in the visual pathway in a rat model of acute intraocular hypertension. Brain Res. 2009;1303:131–143.
7. Gupta N, Ang LC, Noël de Tilly L, et al. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90:674–8.
8. Sponsel WE, Groth SL, Satsangi N, et al. Refined data analysis provides clinical evidence for central nervous system control of chronic glaucomatous neurodegeneration. Trans Vis Sci Technol. 2014 May 6;3(3):1.
9. Frezzotti P, Giorgio A, Motolese I, et al. Structural and Functional Brain Changes beyond Visual System in Patients with Advanced Glaucoma. PLoS ONE. 2014 Aug 27;9(8):e105931. doi:10.1371/journal.pone.0105931
10. Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci 2003;44: 5189–95.
11. Yang D, Fu J, Hou R, et al. Optic neuropathy induced by experimentally reduced cerebrospinal fluid pressure in monkeys. Invest Ophthalmol Vis Sci. 2014;55:3067–73.
12. Wostyn P, De Groot V, Van Dam D, et al. Invest Ophthalmol Vis Sci. 2014;55:5351–2.
13. Berdahl JP, Fautsch MP, Stinnett SS, Allingham RR. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: a case-control study. Invest Ophthalmol Vis Sci. 2008;49:5412–18.
14. Ren R, Jonas JB, Tian G, et al. Cerebrospinal fluid pressure in glaucoma: a prospective study. Ophthalmology. 2010;117:259–66.
15. Wang N, Xie X, Yang D, et al. Orbital cerebrospinal fluid space in glaucoma: the Beijing intracranial and intraocular pressure (iCOP) study. Ophthalmology 2012 Oct;119(10):2065-73.
16. Xie XB, Zhang XJ, Fu J, et al. Noninvasive intracranial pressure estimation by orbital subarachnoid space measurement: the Beijing Intracranial and Intraocular Pressure (iCOP) study. Crit Care 2013 Jul 24;17(4):R162. doi:10.1186/cc12841.
17. Yang D, Fu J, Hou R, et al. Optic neuropathy induced by experimentally reduced cerebrospinal fluid pressure in monkeys. Invest Ophthalmol Vis Sci. 2014;55:3067–73.
18. Jonas JB, Wang NL, Wang YX, et al. Estimated trans-lamina cribrosa pressure difference versus intraocular pressure as biomarker for open-angle glaucoma. The Beijing Eye Study 2011. Acta Ophthalmol. 2014 Jun 24. doi: 10.1111/aos.12480. [Epub ahead of print].
19. Xin C, Zhang W, Wang L, et al. Changes of visual field and optic nerve fiber layer in patients with OSAS. Sleep Breath. 2014 May 8. [Epub ahead of print].
20. Sergi M, Salerno DE, Rizzi M, et al. Prevalence of Normal tension Glaucoma in Obstructive Sleep Apnea Syndrome Patients. J Glaucoma 2007 Jan;16(1):42-46.
21. Lin PW, Friedman P, Lin HC, et al. Normal Tension Glaucoma in Patients With Obstructive Sleep Apnea/Hypopnea Syndrome. J Glaucoma 2011;Dec;20(9):553-558
22. Lee AG, Golnik K, Kardon R, et al. (2002) Sleep apnea and intracranial hypertension in men. Ophthalmology. 2002 Mar;109(3):482-485
23. Wostyn P, Audenaert K, De Dyn PP. Alzheimer’s disease: Cerebral Glaucoma? Medical Hypotheses. 2010 Jun;74(6):973-977
24. Bullock R, Hammond G. Realistic expectations: the management of severe Alzheimer disease. Alzheimer Dis Assoc Disord 2003;17(Suppl. 3):S80–5.
25. 2104 Alzheimer’s Disease Facts and Figures. Available at: http://www.alz.org/downloads/Facts_Figures_2014.pdf (accessed December 13, 2014).
26. Chang LY, Lowe J, Ardiles A, et al. Alzheimer’s disease in the human eye. Clinical tests that identify ocular and visual information processing deficit as biomarkers. Alzheimers Dement. 2014 Mar;10(2):251-61.
27. Guo L, Duggan J, Cordeiro MF. Alzheimer’s Disease and Retinal Neurodegeneration. Current Alzheimer Research. 2010 Feb;7(1):3-14.
28. Stancu IC, Vasconcelos B, Terwel D, Dewachter I. Models of β-amyloid induced Tau-pathology: the long and “folded” road to understand the mechanism. Mol Neurodegener. 2014 Nov 18;9:51.
29. Fagan AM, Perrin RJ. Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomarkers Med. 2012 Aug;6(4):455-476
30. Ohno-Matsu K. Parallel findings in age-related macular degeneration and Alzheimer’s disease. Prog Retin Eye Res. 2011;30:217–238.
31. Albert MS, Dekosky ST, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. AlzheimersDementia 2011 May;7(3):270-9
32. Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986 Aug 21;315(8):485-7
33. Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer’s disease. Ophthalmology 1990 Jan;97(1): 9-17
34. Guo L, Salt TE, Luong V, et al. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci U S A. 2007 Aug 14;104(33):13444-9.
35. Kirbas S, Turkyilmaz K, Anlar O, et al. Retinal nerve fiber layer thickness in Patients with Alzheimer Disease. J Neuroophthalmol. 2013 Mar;33(1):58-61
36. Berisha F, Feke GT, Trempe CL, et al. Retinal Abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci. 2007 May;48(5):2285-9.
37. Kesler A, Vakhapova V, Korczyn AD, et al. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg. 2011;113:523–6.
38. Paquet C, Boissonnot M, Roger F, et al. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2007;Jun 13;420(2):97-99.
39. Iseri PK, Altinas Ö, Tokay T, Yüksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006 Mar;26(1):18-24.
40. Danesh-Meyer HV, Birch H, Ku JY, et al. Reduction of optic nerve fibers in patients with Alzheimer disease identified by laser imaging. Neurology 2006 Nov 28;67(10):1852-4.
41. Kurna SA, Akar G, Altun A, et al. Confocal scanning laser tomography of the optic nerve head on the patients with Alzheimer’s disease compared. Int Ophthalmol 2014 Dec;34(6):1203-11.
42. Inoue T, Kawaji T, Tanihara H. Elevated levels of multiple biomarkers of Alzheimer’s disease in the aqueous humor of eyes with open angle glaucoma. Invest Ophthalmol Vis Sci. 2013 Aug 9;54(8):5353-8.
43. Keenan TDL, Goldacre R, Goldacre MJ. Associations between primary open angle glaucoma, Alzheimer’s disease and vascular dementia: record linkage study. Br J Ophthalmol. 2014 Nov 4;0:1–4 doi: 10.1136/bjophthalmol-2014-305863. [Epub ahead of print]
44. Bach-Holm D, Kessing SV, Mogensen U, et al. Normal tension glaucoma and Alzheimer disease: comorbidity? Acta Ophthalmol 2012 Nov;90(7):683-5
45. Ou Y, Grossman DS, Lee PP, et al. Glaucoma, Alzheimer disease and other dementia: a longitudinal analysis. Ophthalmic Epidemiol 2012 Oct;19(5):285-92.
46. Lin IC, Wang YH, Wang TJ, et al. Glaucoma, Alzheimer’s Disease, and Parkinson’s Disease: An 8-Year Population-Based Follow-Up Study. PLoS ONE 2014 Oct 2;9(9):e108938. doi: 10.1371/journal.pone.0108938.
47. Helmer C, Malet F, et al. Is There a Link between Open-Angle Glaucoma and Dementia?: The Three-City-Alienor Cohort. Ann Neurol 2013;74:171-179.

