Just when you think we’ve discovered everything there is to know about dry eye, new information arises. For instance, the November 2014 Canadian Journal of Optometry included a standalone supplement on “Screening, Diagnosis and Management of Dry Eye Disease.” Also, a Dry Eye Summit took place in December in Dallas. And soon, the Tear Film and Ocular Surface Society will hold a news conference to revisit the exhaustive International Dry Eye WorkShop (DEWS) report, and revamp it with the new knowledge of the day.
So, we still have a good deal to learn.
To that end, here is a recap of what we learned at the 2014 American Academy of Optometry meeting.
Pathophysiology
Many of the scientific papers presented at the Academy meeting added to our understanding of the pathophysiology of dry eye disease. The complexity at a clinical level may be overwhelming. No matter; as eye care clinicians, the better we understand the diseases that we are dealing with, the better we will manage them.
|
|
|
|
|
Researchers recognize a molecular biomarker for Sjögren's-related dry eye. |
• Aqueous deficient vs. evaporative dry eye. Too often, we lump all forms of dry eye together and skip the differentiation of phenotype by ignoring the Schirmer test or phenol red thread test.
One paper differentiated aqueous deficient (ADDE) and evaporative dry eye (EDE) at a molecular level.1 Jillian F. Meadows, OD, and colleagues found that T-helper cells 1, 2 and 17 are active in dry eye disease regardless of etiology. However, ADDE has increased inflammation, as measured by cytokine levels, compared to EDE and mixed disease.
On a practical level, this suggests that topical corticosteroids may be more effective as an initial treatment of ADDE than in other forms of dry eye syndrome.
• Biomarker for Sjögren’s. In earlier work in mice, Nancy A. McNamara, OD, PhD, and colleagues found that the PAX-6 molecule is decreased in Sjögren’s syndrome (SS). PAX-6 regulates stem cells to commit to being corneal cells, thus preventing squamous metaplasia and damage of ocular surface cells from the inflammatory cytokine IL-1β.
In this study, researchers performed impression cytology of the bulbar conjunctiva of human subjects with and without SS to analyze the level of PAX-6.2 This molecule was significantly lower in SS patients and was highly correlated with ocular surface staining. This molecule may serve as a biomarker for SS as well as a target for treatment. Specifically, CD4 T-cells seem to be the main regulators of the loss of PAX-6 in ocular surface cells. Therefore, a topical medication that would change the CD4 T-cell expressions might prevent the loss of PAX-6.
|
|
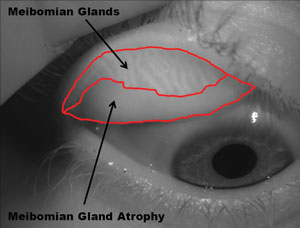
| Contact lenses don't appear to affect meibomian glands. That is, meibomian gland atrophy is no worse in CL wearers than in non-wearers. Photo: Adnrew D. Pucker, OD, MS |
|
• Vitamin D and ocular inflammation. Vitamin D is believed to play a major role in modulating inflammation. In human corneal epithelial cells in vitro, vitamin D lessened inflammation induced from toll-like receptors, which activate immune cell responses. Also, vitamin D influences gene expression to increase proteins that are used in innate immunity.3 This study, by Rose Y. Reins, PhD, and colleagues, furthers our understanding of vitamin D’s protective function, showing that vitamin D is able to diminish inflammation even after removal of the stimulus.
• CLs don’t induce MGD. Meibomian gland disease is an important factor in dry eye, and some evidence suggests that contact lens wearers have greater meibomian gland loss than non-lens wearers.4 As clinicians, we worry about causing harm to our patients, but we also know the value of contact lenses. Two papers put these worries to rest.
Sruthi Srinivasan, PhD, BSOptom, and Andrew D. Pucker, MS, OD, each studied contact lens wearers and non-contact lens wearers and, using meibography, found no difference in meibomian gland dropout in the two groups.5,6
Diagnosis
As an eye care clinician, I’m always interested in diagnostic testing. Diagnosis is a tricky business in clinical practice. There are many questions: Which patients do we test, and which tests do we use? How many tests are needed? Who should have a full tear film and ocular surface disease work-up? What does this cost?
Bear in mind that one recent study found that up to 60% of patients with clinically significant dry eye are asymptomatic.7 This study used osmolarity as the diagnostic criterion for dry eye. Because many of us don’t have the ability to measure osmolarity in our offices, we must use symptoms and other signs, such as staining, to determine dry eye. So, symptoms of any kind do play a big role in clinical practice. We want all of our patients to feel better, so it’s one of the main goals in our treatment plans.
|
|
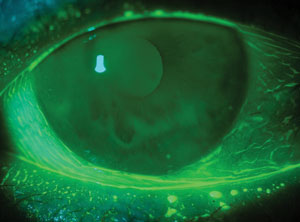
| Look out for conjunctivochalasis, which masquerades as dry eye. Note the redundant folds in an otherwise transparent conjunctiva. Photo: Etty Bitton, OD, MSc |
|
• Do some patients feel more pain? Speaking of symptoms, an interesting paper presented by Eric Li, OD, suggested that we temper our rating of dry eye disease by learning the global pain sensitivity of our patients.8 There is a general pain sensitivity questionnaire that can differentiate those of us who feel pain too much and those who feel pain too little. This understanding may help to explain the very real clinical dilemma of painfully sore eyes with few signs vs. terribly stained ocular surfaces with few symptoms.
• The other reason for dry eye. When a patient presents with ocular discomfort and epiphora, dry eye is usually at the forefront of our differential diagnosis. A poster described two cases presenting with severe dry eye symptoms (OSDI >33/100) including discomfort, tearing and ocular irritation.9 However, full dry eye exams ruled out MGD, lid wiper epitheliopathy and hyperosmolar tear film, but did show shortened tear film breakup time.
The diagnosis: conjunctivochalasis, which is characterized as redundant conjunctival tissue that often presents with symptoms similar to dry eye (including epiphora, foreign body sensation and ocular irritation). Ophthalmic dyes highlight the redundant folds in the otherwise transparent conjunctiva, and can alert us to an early presentation along the lower fornix. So, be sure to add conjunctivochalasis in your differential for dry eye, especially in the presence of normal osmolarity.
Treatment
Our treatment of dry eye is becoming more focused. Anti-inflammatory therapies include pulse-dosed steroids and Restasis (cyclosporine A, Allergan), as well as oral doxycycline, minocycline and azithromycin. It has been suggested recently that Restasis may be more efficacious when used with greater frequency (more than twice daily) in certain patients with severe dry eye.10
Also, azithromycin may be more valuable than tetracycline derivatives in treating meibomian gland disease; it would certainly be a much shorter-term therapy.11,12
|
|
|
|
|
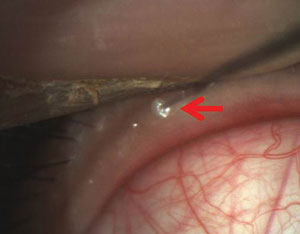
Intraductal probing of blocked meibomian glands can be a more effective treatment than artificial tears in dry eye pati
ents. Photo: Drs. Mickles, Connor, Narayanan et al/University if the Incarnate Word Rosenberg School of Optometry
|
Other interesting treatment research:
• Intraductal meibomian gland probing. Blockage of the meibomian glands is a major cause of increased tear evaporation. So, Srihari Narayanan, OD, PhD, and colleagues presented a poster on intraductal meibomian gland probing (IMGP).13 Their study treated MGD patients with either IMGP or an artificial tear designed to treat evaporative dry eye. They determined that IMGP is more effective than artificial tears alone for MGD. Specifically, subjects in the IMGP group showed more than 50% improvement in symptoms, as well as a doubling of TBUT.
• Phase III results of lifitegrast. An interesting paper by Paul Karpecki, OD, and colleagues reported on Phase III results using lifitegrast as an anti-inflammatory treatment for dry eye.14 Lifitegrast acts as an ICAM-1 decoy, thus preventing some of the cascade of inflammation and initiating treatment further back in the inflammatory process.
In this study, half of the 718 subjects enrolled received topical lifitegrast 5.0% and the other half were given vehicle only. The main outcome measures were inferior corneal staining and reduced symptoms on a visual analog scale. Although there was no difference found in the staining scores, there was significant reduction in dryness symptoms with the use of lifitegrast. The secondary outcomes were total corneal staining, nasal lissamine green staining of the conjunctiva and symptoms. The treatment group had reduced ocular discomfort and decreased itching and foreign body sensation.
An NDA for lifitegrast is expected to be submitted to the FDA early this year. These and other efforts to create unique and better anti-inflammatory drops continue to be an important area of dry eye research.
More to Come
We still have a good deal to learn about dry eye, but we are making progress. The various phenotypes of dry eye are being elucidated. The pathophysiology is better understood. Diagnostic testing is improving, and some of the more innovative tests may become commonly used in private practice. Also, the effort to discover better treatments is well under way.
Meanwhile, we have groups of specialists in North America and around the world that are working to create clinical guidelines and important summaries that will serve to update all of us on evidence-based care and management of dry eye disease.
There are still so many questions. Can we prevent meibomian gland dropout? Are non-preserved drops really better? Is QID better than PRN? Can dry eye disease be a pure neuropathy?
For now, I am determined to take a thorough case history, perform the appropriate testing, consider the inflammatory process associated with dry eye, and to explain the disease clearly to my patients.
Dr. Caffery is in group practice in Toronto, teaches part time at the University of Waterloo School of Optometry, and is secretary-treasurer of the board of the American Academy of Optometry.
|
What’s New in Dry Eye Diagnostic Technology?
• Osmolarity is now used in a number of dry eye practices and, although there is still controversy over its true clinical value, many dry eye experts say that they could not do their job without it. The testing involves a tear sample that is automatically taken up by the tip of the TearLab instrument. The two arms are placed in a well and the osmolarity is read out almost instantly. Normal tears would have an osmolarity of 308mOsml or less. Also, both eyes should have readings that are within 5mOsml of each other. We may need to monitor patients over time to ensure the consistency of the osmolarity. Normal eyes would have little variability over time, but dry eyes would have greater variability both over time and between eyes. • Interferometry, performed by the LipiView instrument, allows us to see the lipid layer moving on the eye and gives us an assessment of the stability of the tear film. It is used for that purpose alone and to decide whether the LipiFlow (TearScience) treatment is recommended. The rough-and-ready clinical test that simulates this stability test is tear break-up time. • Cytokine assays, common in the realm of research, may gain clinical application one day. The hope is that we will be able to take a tear sample, place it in a portable device and determine the exact composition of the tear film, and then customize our treatment for individual dry eye patients. To date, this analysis is limited to the research lab.
However, the enzyme MMP-9 is readily measured in office using the InflammaDry kit. This works and looks like a pregnancy test in that the tear sample delivers a pink line to the stick reader if the enzyme is in a higher concentration than normal. MMP-9 is a useful molecule at a normal level but in higher concentrations can cause intercellular matrices to dissolve on the ocular surface. There is evidence that this enzyme is overproduced by meibomian glands, conjunctival cells and lacrimal glands in dry eye.1-3
• Antibody analysis appears to identify Sjögren’s syndrome-associated dry eye, the most severe form of dry eye disease. Perhaps the biggest problem facing patients with Sjögren’s syndrome is getting a diagnosis. Rheumatology has used blood testing to determine the presence of Sjögren’s syndrome by analyzing many antibodies, including ANA, RF, Anti-Ro/SSA and Anto-La/SSB in serum. Unfortunately, dentists, optometrists, ophthalmologists and family practice physicians often overlook the presenting symptoms of dry eye and dry mouth. Indeed, many patients with “idiopathic” dry eyes have autoantibodies consistent with early SS, at a frequency higher than currently reported in the literature.4 Sjö (Nicox) is test in which a pinprick sample of blood is analyzed for markers of salivary inflammation that may identify Sjögren’s syndrome in the absence of the traditional antibodies alone. The report includes the presence and levels of salivary protein (SP-1) and parotid secretory protein (PSP), which are considered to be early signs of syndrome, as well as other traditional markers. This type of simple in-office lab analysis could offer earlier diagnosis and lead to better management of dry eye and other systemic manifestations in patients with Sjögren’s syndrome.
• Meibography is a fascinating diagnostic tool. It requires expensive instrumentation, but the images that result are clinically important. One can see the number of meibomian glands, the number of dropouts and those that are enlarged easily. Meibomian glands are a very important aspect of dry eye disease, so seeing them clearly is a wonderful clinical tool. For now, we press on the lids and estimate the numbers of glands that are functioning and what the secretions look like.
1. Acera A, Veccino E, Duran JA. Tear MMP-9 levels as a marker of ocular surface inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci. 2013 Dec 23;54(13):8285-91.
|
2. McNamara NA, Gallup M. Establishing PAX6 as a Biomarker to Detect Early Loss of Ocular Phenotype in Human Patients with Sjögren’s Syndrome. Paper presented at American Academy of Optometry Meeting 2014, November 12; Denver, CO.
3. Reins R, McDermott AM, Badour H. Vitamin D Decreases IL-8 Expression after Induction of Inflammation and Influences Gene Expression in Human Corneal Epithelial Cells. Paper presented at American Academy of Optometry Meeting 2014, November 12; Denver, CO.
4. Arita R, Itoh K, Inoue K, et al. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009 Mar;116(3):379-84.
5. Srinivasan S, Pucker AD, Jones-Jordan LA, et al. Meibomian Gland Atrophy Rate in Pre-Presbyopic Contact Lens and Non-Contact Lens Wearers. Paper presented at American Academy of Optometry Meeting 2014, November 15; Denver, CO.
6. Pucker AD, Jones-Jordan LA, Li W, et al. Factors Associated with Meibomian Gland Atrophy in Daily Contact Lens Wearers. Paper presented at American Academy of Optometry Meeting 2014, November 15; Denver, CO.
7. Bron AJ, Tomlinson A, Foulks GN, et al. Rethinking dry eye disease: a perspective on clinical implications. Ocul Surf. 2014 Apr;12(2 Suppl):S1-31.
8. Li W, Graham AD, Lin MC. Understanding Ocular Discomfort and Dryness Using the Pain Sensitivity Questionnaire. Paper presented at American Academy of Optometry Meeting 2014, November 13; Denver, CO.
9. Leger S, Lacroix Z, Bitton E. Conjunctivochalasis: The other reason for dry eye. Poster presented at American Academy of Optometry Meeting 2014, November 13; Denver, CO.
10. Dastjerdi MH, Hamrah P, Dana R. High-frequency topical cyclosporine 0.05% in the treatment of severe dry eye refractory to twice-daily regimen. Cornea. 2009 Dec;28(10):1091-6.
11. Greene JB, Jeng BH, Fintelmann RE, Margolis TP. Oral azithromycin for treatment of meibomitis. JAMA Ophthalmol. 2014 Jan;132(1):121-2.
12. Kashkouli MB, Fazel AJ, Kiavash V, et al. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double-masked open-label clinical trial. Br J Ophthalmol. 2015 Feb;99(2):199-204.
13. Narayanan S, Mickles C, Connor C, et al. Evaporative dry eye treatment with Retaine MGD artificial tears OR intraductal Meibomian gland probing...a comparison. Poster presented at American Academy of Optometry Meeting 2014, November 13; Denver, CO.
14. Karpecki PM, Tauber J, Raychaudhuri A, Semba C. Lifitegrast Ophthalmic Solution 5.0% vs. Placebo for Treatment of Dry Eye Disease: Results of a Phase 3, Randomized, Controlled Trial (Opus-2). Paper presented at American Academy of Optometry Meeting 2014, November 13; Denver, CO.

