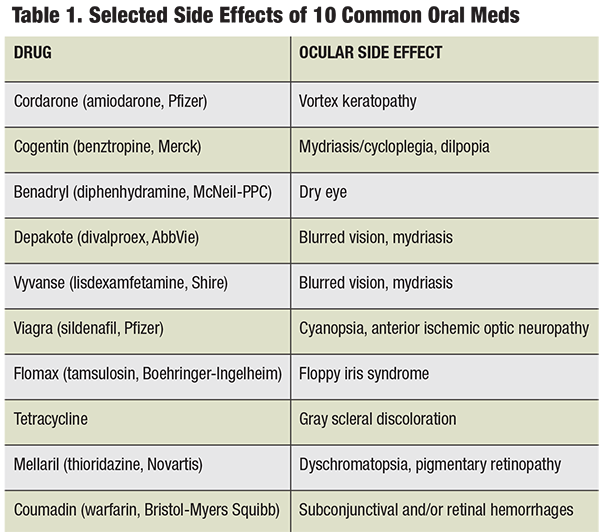
In the age of polypharmacy, many patients present with—quite literally—page-long medication lists. Many commonly prescribed systemic medications can affect the eyes and visual system, with adverse effects ranging from mild dryness to blindness.1 As primary care doctors for the human eye, optometrists often find themselves on the frontlines of health care. This notion, coupled with the reality of an aging patient population makes it paramount that optometrists remain cognizant of the potential side effects associated with the common systemic medications; several of the most commonly used medications are discussed here.
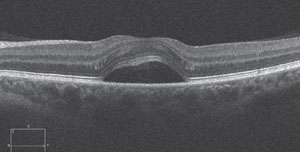 | |
| Fig. 1. SD-OCT scan of a 40-year-old female with corticosteroid-induced CSR. The patient presented sudden onset of unilateral blur several weeks after being prescribed oral prednisone for allergies. CSR resolved subsequent to steroid cessation. |
Corticosteroids
As the standard for treatment of inflammation, corticosteroids such as prednisone, methylprednisolone, hydrocortisone, dexamethasone, etc., are used for a wide variety of acute and chronic conditions, such as arthritis, inflammatory bowel disease (IBD) and sarcoidosis. These diseases typically mandate long-term treatment and may persist for many years. Even at relatively low doses, long-term treatment with systemic corticosteroids carries risks for several adverse effects.2
The formation of posterior subcapsular cataract (PSC) and ocular hypertension are arguably the two most well known effects of both systemic and topical corticosteroid therapy. Research shows corticosteroids dysregulate blood glucose levels, which may cause ophthalmic manifestations such as induced myopia in the presence of diabetes; however, central serous retinopathy (CSR) is also a well-documented adverse ocular effect of corticosteroid use.3,4 Steroid-induced CSR and steroid-induced ocular hypertension both tend to resolve when the patient discontinues the medication.
Anti-Hypertensives
Systemic hypertension is among the most common chronic diseases in the United States, and the frequency at which anti-hypertensive medications appear on patients’ medication lists follow suit.5 Three of the most common types of hypertension medications are beta-blockers, diuretics and angiotensin-converting enzyme (ACE) inhibitors. Common beta-blockers include atenolol, metoprolol and carvedilol. Just as topical beta-blockers suppress aqueous humor production, they may also suppress tear production, potentially leading to symptomatic dry eye disease.6,7 Conjunctival inflammation may also occur or worsen with beta blocker use. Diuretics, such as hydrochlorothiazide, furosemide and triamterene, can also cause or exacerbate dry eye disease.8 Uncommonly, supportive therapy may not be enough to quell the dry eye, and changing to another class of anti-hypertensive therapy may be necessary.
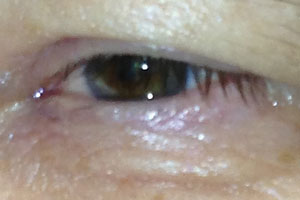 | |
| Fig. 2. A 64-year-old white male with mild conjunctival inflammation, angioedema of the eyelids associated with a recent increase in lisinopril dosage. |
Common ACE inhibitors include lisinopril, benazepril and enalapril. Conjunctival inflammation with or without angioedema of the eyelids has been reported with ACE inhibitor use. Figure 2 is the left eye of a 64-year-old male who presented with conjunctival inflammation and angioedema of both eyelids, which worsened around the same time that his lisinopril (a commonly prescribed ACE inhibitor) dosage was increased from 5mg to 10mg QHS. Interestingly, his ocular condition did not readily respond to topical corticosteroid therapy, but his symptoms did subside with his medication being changed to Cozaar (losartan potassium, Merck), an angiotensin II receptor antagonist, after a brief conversation with the prescribing doctor.
This case highlights the potential for adverse reactions even at relatively low concentrations.
Statins
Hypercholesterolemia is a major contributor to cardiovascular disease (one of the leading causes of death worldwide).9 Statins, such as atorvastatin, lovastatin, and simvastatin, work well to reduce elevated cholesterol levels, and their side-effect profiles are relatively desirable—their systemic side effects are not as common as with other medications.
Commonly, muscle weakness or soreness may occur with statin use, which researchers attribute to induced mitochondrial dysfunction at the cellular level.10 Myopathic symptoms may present to the optometrist first as diplopia, ptosis or soreness around the eyes. When new-onset myopathic symptoms occur in the presence of statin use, the statin should be considered a potential cause, and the prescriber should be made aware so that changes to therapy can be made if need be. Finally, cataract formation has been reported as an uncommon side effect of statin use.
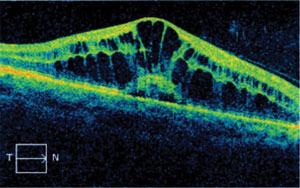 | |
| Fig. 3. Drug-induced macular edema is uncommon, but a potentially sight-threatening side effect of fingolimod therapy. |
In recent years, investigators have studied the possibility of statin use having a protective effect against the development of open-angle glaucoma.11,12 There is not enough evidence to change current treatment modalities, but this research will likely continue as the race to find effective so-called “neuroprotective” therapeutic agents for glaucoma continues.
Multiple Sclerosis Medications
Several years ago, Gilenya (fingolimod, Novartis) became the first FDA-approved oral medication for relapsing-remitting multiple sclerosis (MS), the most common form of MS. In recent years, Gileyna has been implicated in the formation of macular edema by increasing vascular permeability.14-16 For this reason, it is recommended that patients have a baseline ophthalmic examination, including dilated fundus evaluation, and a second examination three to four months subsequent to the first, since studies indicate that adverse effects tend to manifest after four months.17 OCT studies are highly beneficial in establishing a baseline and monitoring patients who are taking Gilenya. Patients should be counseled on the risks associated with Gilenya (e.g., macular edema), and advised to monitor their vision at home and to call immediately if they experience changes in vision. The edema is thought to resolve, in most cases, with cessation of the medication.15 Treatment of the edema may be indicated for recalcitrant cases or where the positive effects of this medication are too great to warrant its discontinuation. Although macular edema is not a common side effect of fingolimod therapy, communication regarding these recommendations should be established and maintained with the prescribing doctor.
Migraine Medications
Topamax (topiramate, Janssen Pharmaceuticals) has been widely prescribed for years to prevent the occurrence of migraine headaches. The development of acute angle closure glaucoma is an uncommon, but well-documented side effect of this medication.18-20 Research shows the risk of topiramate-induced angle closure is five times greater in persons under the age of 50 and is typically bilateral (in contrast to angle closure resulting from pupillary block).18 Since the cause of topiramate-induced angle closure is edema and effusion of the uvea, pilocarpine should be avoided.
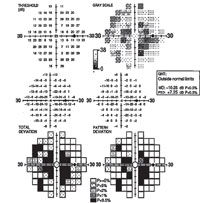 | |
| Fig. 4. The visual field study of a 52-year-old black female who presented with central scotomas, which was associated with methotrexate-induced toxic optic neuropathy. |
In addition to lowering IOP in the acute phase of the attack, cycloplegics and topical steroids are also indicated to reduce vascular permeability within the uvea itself. Peripheral iridotomy is of no use, as primary pupillary block is not the cause of topiramate-induced angle closure. Myopic shifts may occur as a result of the same mechanism as the angle closure. Acute-onset maculopathy and transient, non-specific visual field defects have also been reported.
DMARDs
Disease-modifying anti-rheumatic drugs, or DMARDs, is a clinically descriptive term designating a family of medications whose therapeutic effects were first associated with rheumatoid arthritis. In fact, DMARDs are used to treat other inflammatory/autoimmune diseases as well, such as psoriasis and Crohn’s disease.21 The member of the DMARD family that is most well-known for its ocular side effects is the antimalarial drug Plaquenil (hydroxychloroquine, Sanofi Canada). Routine evaluations for its characteristic bull’s eye maculopathy, and daily visual monitoring on the part of the patient are necessary to prevent this permanent and potentially visually-impairing sequela.
 |
Fundus photography, visual field studies, fundus autofluorescence and multifocal electroretinogram (mfERG) studies have all shown value in detecting bull’s eye maculopathy. In the past decade or so, SD-OCT studies have become popular as a means of objectively assessing early retinal damage with a high resolution on the order of just a few microns. In 2011, SD-OCT was added to the American Academy of Ophthalmology’s clinical guidelines for detecting hydroxychloroquine and chloroquine toxicity.22
Trexall (methotrexate, Barr Laboratories) was first developed as a form of chemotherapy and continues to be used as such; however, this drug has gained popularity as an effective DMARD agent.23 It has been shown to cause certain ocular side effects even at low doses (a typical dose may be 2.5mg orally two to three times per week), the most significant of which is toxic optic neuropathy (see Figure 4).24 Folic acid supplementation may prevent or lessen some side effects of methotrexate and other DMARDs.25
Truly, no medication is without some side effect. However, keeping abreast of the most common ocular side effects of the most commonly prescribed systemic medications will aid in correct and timely diagnoses for your patients, especially since signs and symptoms such as redness, dryness and visual disturbances are often quickly attributed to and treated as sequelae of primary eye conditions.
As well, communication with the prescribers of these medications is important in relaying the fact that, as primary care doctors for the human eye, we, as optometrists, are monitoring for these adverse effects, some of which can be visually devastating if not diagnosed and treated early on.
Dr. Casella is the owner of a third-generation private practice in Augusta, GA. He has no financial interest in any of the products mentioned in this article.
1. Passman RS, Bennett CL, Purpura JM, et al. Amiodarone-associated optic neuropathy: a critical review. Am J Med. 2012 May;125(5):447-53.2. Alderaan K, Sekicki V, Magder LS, Petri M. Risk factors for cataracts in systemic lupus erythematosus (SLE). Rheumatol Int. 2015 Apr;35(4):701-8.
3. Kappe C, Fransson L, Wolbert P, et al. Glucocorticoids suppress GLP-1 secretion: possible contribution to their diabetogenic effects. Clin Sci (Lond). 2015 Sep;129(5):405-14.
4. Ricketti PA, Unkle DW, Cleri DJ, et al. Central serous chorioretinopathy secondary to corticosteroids in patients with atopic disease. Allergy Asthma Proc. 2015 Mar-Apr;36(2):123-9.
5. Gillespie CD, Hurvitz KA. Prevalence of hypertension and controlled hypertension—United States, 2007–2010. CDC Supplements. 2013 Nov;62(3):144-48.
6. Rittenhouse KD, Pollack GM. Pharmacodynamics of beta-blocker modulation of aqueous humor production. Exp Eye Res. 2000 Apr;70(4):429-39.
7. Mosseri M, Ben-Ishay D. Beneficial effect of pindolol in keratoconjunctivitis sicca induced by propranolol and atenolol. Isr J Med Sci. 1988 Apr-May;24(4-5):259-60.
8. Bergmann MT, Newman BL, Johnson NC Jr. The effect of a diuretic (hydrochlorothiazide) on tear production in humans. Am J Ophthalmol. 1985 Apr 15;99(4):473-5.
9. Fauci AS, Touchette NA, Folkers GK. Emerging Infectious diseases: a 10-Year perspective from the national institute of allergy and infectious diseases. Emerg Infect Dis. 2005 Apr;11(4):519-25.
10. Golomb BA, Evans MA. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8(6):373–418.
11. Stein JD, Newman-Casey PA, Talwar N, et al. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012 Oct;119(10):2074–81.
12. Marcus MW, Müskens RPHM, Ramdas WD, et al. Cholesterol-lowering drugs and incident open-angle glaucoma: a population-based cohort study. PLoS One. 2012;7(1):e29724.
13. Goldenberg MM. Multiple Sclerosis Review. PT. 2012 Mar;37(3):175–84.
14. Ontaneda D, Hara-CleaverC, Rudick RA, et al. Early tolerability and safety of fingolimod in clinical practice. J Neurol Sci. 2012 Dec 15;323(0):167–72.
15. Nolan R, Gelfand JM, Green AJ. Fingolimod treatment in multiple sclerosis leads to increased macular volume. Neurology. 2013 Jan 8;80(2):139–144.
16. di Nuzzo L, Orlando R, Nasca C, et al. Molecular pharmacodynamics of new oral drugs used in the treatment of multiple sclerosis. Drug Des Devel Ther. 2014;8:555–68.
17. Jain N, Bhatti MT. Fingolimod-associated macular edema: incidence, detection and management. Neurology. 2012;78:672-80.
18. Symes RJ, Etminan M, Mikelberg FS. Risk of angle-closure glaucoma with bupropion and topiramate. JAMA Ophthalmol. 2015 Oct 1;133(10):1187-9.
19. Grewal DS, Goldstein DA, Khatana AK, et al. Bilateral angle closure following use of a weight loss combination agent containing topiramate. J Glaucoma. 2015 Jun-Jul;24(5):e132-6.
20. Murphy RM, Bakir B, O’Brien C, et al. Drug-induced bilateral secondary angle-closure glaucoma: a literature synthesis. J Glaucoma. 2015 Apr 21. [Epub ahead of print].
21. Lorenzcorresponding H, Kalden JR. Perspectives for TNF-α-targeting therapies. Arthritis Res. 2002; 4(Suppl 3):S17–S24.
22. Marmor MF, Kellner U, Lai TY, et al. American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118(2):415–22.
23. Tarnowski M, Paradowska-Gorycka A, Dabrowska-Zamojcin E, et al. The effect of gene polymorphisms on patient responses to rheumatoid arthritis therapy. Expert Opin Drug Metab Toxicol. 2015 Nov 26. [Epub ahead of print].
24. Clare G, Colley S, Kennett R, et al. Reversible optic neuropathy associated with low-dose methotrexate therapy. J Neuroophthalmol. 2005 Jun;25(2):109-12.
25. Patel V, Wang Y, MacDonald JK, et al. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014 Aug 26;8:CD006884.

