 A 67-year-old Hispanic male presented for a routine visit. He was concerned that he might have cataracts and wanted a thorough examination. He enjoyed good vision all his life, and reported that his eyes were functioning well. He denied any history of ocular injury, surgery or infection. His medical history was remarkable for hypertension.
A 67-year-old Hispanic male presented for a routine visit. He was concerned that he might have cataracts and wanted a thorough examination. He enjoyed good vision all his life, and reported that his eyes were functioning well. He denied any history of ocular injury, surgery or infection. His medical history was remarkable for hypertension.
On examination, confrontation visual fields were full to careful finger counting O.U. Pupils were equally round and reactive, with no afferent defect. Ocular motility testing was normal.
His anterior segments were remarkable for peripheral cortical lens changes and grade 1+ nuclear sclerotic cataracts O.U. His IOP measured 15mm Hg O.U.
Dilated fundus exam revealed a clear vitreous as well as moderate-sized cups with good rim coloration and perfusion O.U. We noted obvious pathologic changes located in the macula and posterior pole of the right eye (figure 1). The left eye, however, was completely normal. We also obtained spectral domain optical coherence tomography (SD-OCT) scans of both eyes (figures 2 and 3).
Take the Retina Quiz
1. How would you classify the changes seen in the macula?
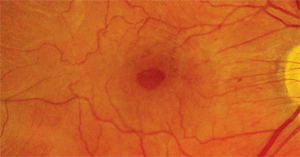
1. The fundus photograph of our patient’s right eye. Note the changes in the macula. What would you expect his visual acuity to measure in this eye?
a. Full-thickness macular hole.
b. Epiretinal membrane with a pseudohole.
c. Epiretinal membrane with a lamellar hole.
d. Epiretinal membrane with a full-thickness hole.
2. What would you expect the patient’s visual acuity to be in his right eye?
a. 20/25 to 20/30.
b. 20/50 to 20/60.
c. 20/100 to 20/200.
d. 20/400.
3. How would you interpret the foveal changes seen on SD-OCT?
a. Lamellar macular hole (LMH).
b. Macular pseudohole (MPH).
c. Full-thickness hole.
d. Cystoid macular edema.
4. How should we manage this patient?
a. Observation.
b. Pars plana vitrectomy and membrane peel.
c. Macular hole surgery.
d. Intravitreal Avastin (bevacizumab, Genentech/Roche) injection.
For answers, see
below.
Discussion
Examination of the right macula and posterior pole revealed a very prominent epiretinal membrane. This was seen as a glisteny, refractile, fibrocellular membrane on the surface of the retina that was causing dragging and tortuousity of the retinal vessels. Further, we noted an obvious hole located directly in the center of the macula. On stereoscopic examination, the hole exhibited substantial depth. In fact, the entity was so deep that we were convinced it could not be anything but a full-thickness macular hole.
Alas, looks can be deceiving. Despite its clinical appearance, the entity was not a full-thickness macular hole.
Full-thickness holes tend to be round and include a surrounding cuff of fluid that represents a small neurosensory retinal detachment. Our patient’s macular hole was more elliptical than round and did not exhibit a cuff of fluid. Also, most full-thickness holes have yellow-white deposits at their base. Again, we did not detect such deposits in our patient. Finally, patients with full-thickness holes usually have an acuity of 20/60 to 20/200, depending on the size and duration of the hole. Amazingly, our patient’s acuity was 20/25 O.D.
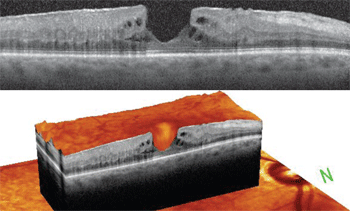
2, 3. Cross-section (top) and 3-D SD-OCT (bottom) scans of the right macula. What changes do you note, both laterally and at the base of the macula?
But, if it’s not a full-thickness macular hole, what is it? Unfortunately, that’s not such an easy question to answer. Given the presence of the epiretinal membrane, we initially assumed that he had an MPH. An MPH is nothing more than a hole within an epiretinal membrane that develops secondary to centripetal contraction.
On SD-OCT, a full-thickness macular hole exhibits an anvil- shaped configuration as well as a full-thickness defect and loss of the photoreceptor elements. Our patient’s SD-OCT scan clearly shows that this was not a full-thickness defect.
Also, there was obvious steepening of the foveal depression walls, which––when combined with the epiretinal membrane––was highly indicative of an MPH.
So, our patient has an MPH, right? It’s possible, but here is where it gets tricky. The SD-OCT also suggests that this, in fact, may be an LMH.
LMHs are partial-thickness holes that develop as a result of the abortive process of a full-thickness macular hole during the early stages of formation.1,2 Understandably, these are quite rare and are almost never seen. In fact, before the development of OCT, it’s hard to imagine how a diagnosis of an LMH could ever be confirmed. OCT has greatly helped in differentiating and understanding these macular lesions; however, LMHs still remain a bit of a mystery, because the features of MPHs and LMHs sometimes overlap.
On OCT, both MPHs and LMHs have an irregular foveal contour and wall steepening in the absence of a full-thickness defect with intact photoreceptors. But, the presence of an intraretinal schisis between the inner and outer retinal layers is what differentiates an LMH from an MPH. This finding is thought to represent a split between the outer plexiform layer and the outer nuclear layer, and appears as a horizontal or lateral extension near the base of the LMH on OCT.2-4
In our patient, the SD-OCT revealed a fairly deep defect with some obvious cystoid spaces seen anteriorly along the walls of the hole. However, there appeared to be more cystoid spaces located posteriorly at the base of the hole. These were not true cystoid spaces, but rather schisis cavities that were developing from intraretinal splitting likely due to tractional forces caused by the epiretinal membrane.
It is important to note that the epiretinal membrane was causing this defect, because it was traditionally believed that MPHs and LMHs were completely separate entities with different etiologic factors. However, it is now recognized that MPHs can evolve into LMHs, and that the causative tractional forces can be the same.3
Because our patient’s visual acuity was so good, I elected to observe him. He returned six months later without any symptoms or changes in his acuity. We repeated SD-OCT testing, which showed no anatomic changes. We will continue to monitor his progress.
1. Gass JD. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment, vol. 2. St. Louis: C.V. Mosby Company; 1987:671-94.
2. Chen JC, Lee LR. Clinical spectrum of lamellar macular defects including pseudoholes and pseudocysts defined by optical coherence tomography. Br J Ophthalmol. 2008 Oct;92(10):1342-6.
3. Michalewski J, Michalewska Z, Dziegielewski K, Nawrocki A. Evolution from macular pseudohole to lamellar macular hole – spectral domain OCT study. Graefes Arch Clin Exp Ophthalmol. 2011 Feb;249(2):175-8.
4. Witkin AJ. Redefining lamellar holes and the vitreomacular interface: An ultrahigh resolution optical coherence tomography study. Ophthalmology. 2006 Mar;113(3):388-97.
Answers to the Quiz
1. c
2. a
3. a
4. a

