Irreversible tissue injury and death can occur within three hours of a stroke, so time is of the essence in getting these patients the urgent attention they need in a hospital setting.
Better yet, we need to talk to our patients about stroke before we reach this point. As primary eye care providers, are we educating our patients appropriately and adequately regarding stroke risk and its signs and symptoms? Let’s take a look at some of the warning signs that we should be relaying to our patients, as well as how stroke correlates to the eye specifically.
 Blood Flow, Interrupted
Blood Flow, Interrupted
A stroke (more formally called a cerebrovascular accident, or CVA) is caused by an interruption in blood flow through the brain. Ischemic strokes, which account for approximately 87% of cases, occur when a blood vessel is obstructed by a blood clot or embolus that came from a different location.1 Typical treatment involves a tissue plasminogen activator (TPA)—a “clot buster.”
Hemorrhagic strokes, on the other hand, usually stem from a rupture of a weakened blood vessel, such as with an aneurysm or arterial-venous malformation. The leaked blood compresses adjacent areas and tissue, and can lead to a painful headache, nausea and vomiting. (These symptoms can occur with an ischemic stroke, but are more likely to present with a hemorrhagic stroke.)
The scary fact is patients who suffer from a stroke often do not recognize that they are having a stroke, nor do they seek treatment or medical attention in a timely fashion. According to one study, only 22% of stroke victims who called for an ambulance recognized the problem was a stroke and called within one hour of symptom onset.2
This is why it’s crucial to educate patients about the common clinical symptoms of stroke, including:
• Weakness or paralysis on one side of the body (face, arm, leg).
• Slurred speech.
• Confusion.
• Loss of balance.
• Tingling, burning or numbness of the skin.
• Headache.
• Vision loss.
Risk factors for stroke include, but are not limited to, family history, age (>55 years), race (African Americans have the highest risk), sex (male), hypertension, diabetes, high cholesterol, cigarette smoking, cardiovascular disease, obesity, sleep apnea, atrial fibrillation and giant cell arteritis. So it’s particularly important to talk with patients who fall within one or more of those categories.
In addition, one of the most important risk factors for a stroke is a transient ischemic attack (TIA) or mini-stroke. (See “‘Mini-strokes’ Are a Significant Warning Sign.”)
Evidence shows education does make a difference. Survey participants reported that the knowledge that stroke is serious and treatable is what prompted them to call 9-1-1 and get help.2,3
 Transient Monocular Vision Loss
Transient Monocular Vision Loss
Several studies have identified transient monocular vision loss (TMVL) as the single greatest predictor of hemodynamically significant stenosis on a carotid ultrasound.4-6 Any time that a patient reports TMVL, consider the possibility that carotid artery disease is responsible. Patients tend to report painless total or sectoral loss of vision lasting from a few seconds to a few hours, which resolves completely. During an eye exam, you may find an explanation for the transient vision loss; however, the patient’s ocular health may also be unremarkable.
In any case of TMVL, consider the patient’s overall health and order a carotid ultrasound to evaluate the patient for significant blockage. Perhaps even more important, while the patient is still in the exam chair, it is our responsibility as primary eye care providers to ask pertinent questions to rule out a possible TIA. (See “Act FAST When You See Symptoms,” page 40.) Because symptoms of TIA mirror those of stroke, it is also possible that these questions may help you diagnose an acute stroke.
The difficult part in this triage process is determining whether the patient needs to go to the emergency room immediately or if he or she can schedule an appointment with their primary care provider in a few days.
You don’t want to unnecessarily worry your patients, but you also don’t want to mismanage a patient and risk permanent neurologic damage.
Carotid Artery Disease
Many eye findings that worry optometrists about stroke risk often relate to the carotid artery—either as a source of plaque (retinal emboli, artery occlusion) or as a result of significant carotid artery blockage (venous stasis retinopathy, TMVL). Carotid artery stenosis is a major cause of morbidity and mortality, and the eye may be the first place it manifests.
While TMVL is a symptom that may prompt a patient to seek medical care, venous stasis retinopathy (VSR) may not have any significant symptoms and routine eye exams are crucial for its detection.
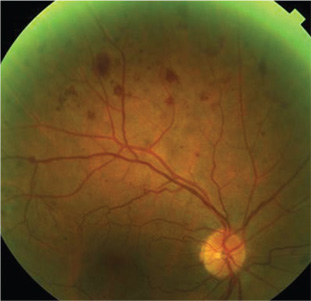 |
| 1. Mid-peripheral hemorrhages, as seen
here, may be a sign of carotid artery disease and may prompt further testing. |
When hemorrhages are seen outside the posterior pole, carotid artery disease should be considered as a potential underlying cause. Specifically, the study found that 69% of patients with VSR had between one to five mid-peripheral hemorrhages per quadrant.7 Thus, from a management standpoint, these are individuals who should have a carotid ultrasound ordered to detect hemodynamically significant stenosis and educated regarding stroke risk.
Ocular ischemic syndrome (OIS) is similar to VSR, except the anterior segment is also affected by carotid artery blockage and severe chronic panocular vascular compromise. Because of its direct link to carotid stenosis, individuals with OIS are at risk for TMVL and TIA or may have already suffered a previous episode.8 The OIS triad of findings includes mid-peripheral hemorrhages, dilated retinal veins and iris neovascularization. In one study, 67% of OIS patients presented at their initial examination with neovascularization of the iris.9
Individuals with OIS can experience progressive vision loss from macular edema and/or ischemia as well as complications from neovascularization of the disc, retina, iris and angle. In particular, OIS patients are at high risk for neovascular glaucoma.
One study found that 35% of patients have neovascularization of the iris and IOP greater than 22mm Hg at presentation.10 In addition to iris and angle neovascularization, anterior segment involvement in OIS patients may include corneal edema, anterior uveitis and conjunctival injection.
In one study, the five-year mortality rate of patients with OIS was found to be 40% compared to 11% of controls.8 Specifically, 63% died of cardiac disease while 19% died from stroke.8 These study results suggest that individuals with OIS would benefit from education about the signs of stroke as well the urgency with which it needs to be addressed.
In addition to educating patients regarding stroke, eye findings consistent with carotid artery disease should also prompt you to order a carotid ultrasound to evaluate for hemodynamically significant stenosis (figure 1). Typically, a 60% to 70% blockage may necessitate a vascular consult to see if the patient is a good candidate for carotid artery surgery. If a patient is not found to have significant carotid artery blockage, but is symptomatic for TMVL or TIA, consider a consult sooner.
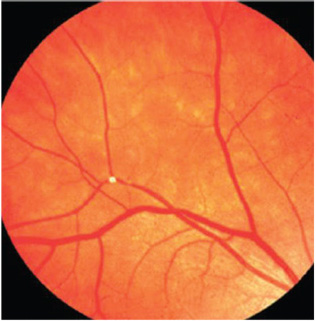 |
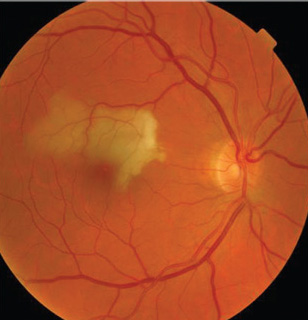 |
| 2. In the Beaver Dam Eye Study, the
presence of retinal emboli was shown to be associated with a threefold greater risk of fatal stroke over eight years.18 |
3. A study in Taiwan found retinal arterial
occlusions increase risk of subsequent stroke, particularly within the first six months following the occlusion.16 |
Retinal emboli are another risk factor for stroke that requires proper education and work-up in our patients. Emboli can be asymptomatic, but can also cause more severe visual sequelae in cases of retinal artery occlusion. Most emboli are either cholesterol, calcific or fibrino-platelet in nature, with the majority being of cholesterol composition. You should order proper imaging to evaluate the carotid artery and the heart as the source of the retinal emboli. One study found that there was an abnormal echocardiogram in 62% of central retinal artery occlusions (CRAO) and 44% of branch retinal artery occlusions (BRAO).11 Similarly, there was plaque present on carotid doppler in 71% of CRAOs and 66% of BRAOs.11
Cholesterol or Hollenhorst plaques tend to be refractile, yellow or white in color, round in shape, and found at a retinal arterial bifurcation. They originate in the carotid arteries and tend to be transient in nature. In the Beaver Dam Eye Study, 90% of them disappeared over a five-year period.12 In addition, cholesterol plaques tend to be associated with TMVL rather than permanent vision loss.13
Dr. Robert Hollenhorst, in his landmark study on cholesterol plaques, found that 15% of patients who had the plaques died within one year, 29% died by year three and 54% died within seven years.14 In addition, more than one-third of patients developed stroke or suffered from TIA during the study follow-up.14 Fortunately, medications and treatments have improved since 1966, but the study still shows that cholesterol plaques are a sign of an unhealthy cardiovascular system and increase the risk for stroke.
Calcific emboli tend to originate from damaged heart valves. Specifically, calcific aortic stenosis and mitral/aortic valve disease can lead to the flat, white, non-refractile emboli we see in the eye. These emboli tend to become lodged at blood vessels of the optic nerve and are more likely to lead to permanent vision loss.13 Fibrino-platelet plaques can originate from both the carotid artery and the heart. They tend to be dull gray-white plugs that are long in shape and smooth in appearance. Although typically mobile, these plaques can cause retinal arterial occlusions when they become stuck or lodged within a vessel.13
With regards to stroke risk, the Beaver Dam Eye Study found that retinal emboli were associated with a threefold greater risk of fatal stroke over eight years (figure 2).12 The Blue Mountain Eye Study found that over 12 years, 30% of patients with retinal emboli died—with 4% of the deaths resulting from stroke. There was a moderate threefold increase in stroke-related mortality rates in patients with emboli.15 In general, patients with emboli have a higher mortality rate compared to those without emboli.
Studies have also shown that patients with retinal arterial occlusion (RAO) are also at higher risk for stroke. Patients with RAO have a higher prevalence of TIA as well as stroke.11 Recently, a population-based study in Taiwan found that 19.6% of RAO patients went on to suffer a stroke, while only 10% of control group patients suffered a stroke (figure 3).16
Stroke risk was particularly high in the first month following the artery occlusion and most strokes occurred within six months, suggesting the need to educate patients at the time of the retinal finding. The study also found that patients with CRAO had higher rates of stroke than patients with BRAO.16
In the presence of retinal emboli, with or without artery occlusion, optometrists need to properly educate patients about their increased stroke risk and the need for urgent medical attention if they experience them. Moreover, order a carotid doppler and echocardiogram to find the source of the emboli and refer appropriately with detection of any abnormalities to the appropriate specialist.
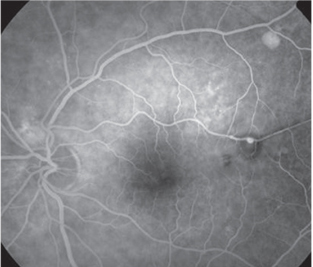 |
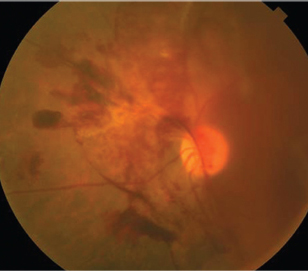 |
| 4. Fluorescein angiography illustrating a retinal
arterial macroaneurysm (RAM), resulting from hypertension. Uncontrolled blood pressure can increase the risk of stroke four- to sixfold. |
5. In the Wisconsin Epidemiological Study in
Diabetic Retinopathy, the risk of stroke was found to be six times higher in patients who had proliferative diabetic retinopathy.26 |
Elevated blood pressure can increase the risk of stroke through atherosclerosis of the vessels over time; this may lead to blockage of small vessels in the brain and ischemia.
Hypertension can also weaken blood vessels and cause an aneurysm, which can disturb blood flow. In the eye, early microvascular changes from hypertension, such as arteriolar narrowing, artery-vein nicking and artery opacification, have been shown to increase the risk of stroke. In the Atherosclerosis Risk in Communities (ARIC) Study, artery-vein nicking and artery narrowing were shown to increase the odds of MRI-defined subclinical infarction by nearly twofold.17
As the severity of hypertension increases, there can be further breakdown of the blood-retina barrier and “moderate” findings, such as flame-shaped hemorrhages, cotton-wool spots and exudates. Similarly, cotton-wool spots and hemorrhages have been shown to increase relative risk of incident stroke two to three times compared to individuals without these retinal findings.18
“Severe” hypertensive retinopathy can occur when elevated blood pressure causes an increase in intracranial pressure and optic nerve swelling. Severe hypertension can also lead to infarction of segments of the choriocapillaris. Siegrist’s streaks refer to linear RPE hyperplasia over infracted choroidal arterioles, and Elschnig spots are a sign of non-perfused choriocapillaries. Uncontrolled hypertension has been shown to increase the risk of stroke four- to sixfold, so this is another group we need to educate (figure 4).
Hypertension can also lead to retinal-arterial macroaneurysms. Elevated blood pressure can cause the smooth muscle lining arteries to be replaced with collagen, making the arterial wall less elastic and more susceptible to aneurysm formation. This is typically found in older female patients and is associated with high blood pressure in up to 79% of cases.19
Diabetes
Diabetes increases the risk for stroke by inhibiting blood flow and fostering ischemia. In the eye, diabetic retinopathy is commonly detected in individuals who have poorly controlled blood sugars or have had the disease more than 10 years. The ARIC study found the risk of incident ischemic stroke was two to three times higher in individuals with non-proliferative diabetic retinopathy vs. individuals without diabetic retinopathy.17 In addition, the level of diabetic retinopathy also seemed to correlate with stroke risk. Of the 1,305 individuals in the study who had no diabetic retinopathy, only 3.9% went on to suffer an ischemic stroke. In contrast, 9.6% of individuals with mild to moderate diabetic retinopathy and 11.4% of individuals with severe diabetic retinopathy went on to suffer a stroke.20
Diabetics who have proliferative diabetic retinopathy (PDR) are not only at risk for devastating ocular sequelae if not properly treated, they are also at increased risk for stroke. The Wisconsin Epidemiological Study in Diabetic Retinopathy found the risk of the stroke was six times higher in patients with PDR, and the risk of stroke mortality was double compared to patients without PDR (figure 5).21,22
Asymmetric diabetic retinopathy is found in approximately 5% to 10% of diabetic patients and can also be a sign of carotid disease and increased stroke risk.23,24 In previous studies, “asymmetry” has been defined as PDR in one eye with no retinopathy in the fellow eye, or as a two to three grade difference between the eyes. The literature has been somewhat inconsistent. In the seminal study on this topic, Andrew Gay, MD, and Arthur Rosenbaum, MD, found that in the majority of their subjects, severe carotid stenosis was found ipsilateral to the eye with less retinopathy.25 They theorized that carotid disease retards progression of retinopathy in the ipsilateral eye or accelerates it in the contralateral eye.25
Since then, two significant studies have found that the connection is not that definitive. The Duker group found that it was “50/50” whether the ipsilateral or contralateral eye with PDR had more severe carotid stenosis.23 They argued that the technique used in the Gay study, ophthalmodynamometry, was not direct evidence of carotid stenosis.23 Their findings were supported by another study from Japan, which proposed that ocular ischemic syndrome can be additive to diabetic retinopathy and that PDR is more likely to be on the same side as the carotid stenosis in cases of asymmetric retinopathy.26
 Case-by-Case Basis
Case-by-Case Basis
While ocular manifestations of carotid artery disease (TMVL, VSR, OIS, retinal emboli) have a clear connection with increased future stroke risk and warrant patient education in almost all cases, the need to educate patients with microvascular signs (diabetic and hypertensive retinopathy) may not be as clear for some practitioners.
While artery-vein nicking and mild non-proliferative diabetic retinopathy have been shown to increase risk for future stroke in certain studies, if the blood pressure and/or blood sugars are well-controlled, some ODs may not feel compelled to educate their patient regarding stroke. Instead, they may elect to educate the patient on blood sugar and/or blood pressure control, which is likely adequate for low-risk patients.
Ultimately, the need to educate our patients about stroke risk and signs of stroke should be taken on a patient-by-patient basis and depends on patient personality as well as doctor comfort. Older patients with multiple vasculopathies who are not compliant with their medications are likely better candidates for education compared to relatively younger and healthier individuals who are more compliant with their treatments.
Overall, the role of the primary care optometrist can be paramount in detecting current stroke as well as preventing future incidents. With the majority of at-risk patients poorly educated about the signs of stroke as well as the need for urgent care when it occurs, we can help our patients to recognize these signs and to act appropriately.
Dr. Chu practices at the Salisbury VA Medical Center in Salisbury, NC.
1. Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcomittee. Circulation. 2008 Jan 29;117(4):e25-2146.
2. Mosley I, Nicol M, Donnan G, et al. Stroke symptoms and the decision to call for an ambulance. Stroke. 2007 Feb;38(2):361-6.
3. Mikulík R, Bunt L, Hrdlicka D, et al. Calling 911 in response to stroke: a nationwide study assessing definitive individual behavior. Stroke. 2008 Jun;39(6):1844-9.
4. Bull DA, Fante RG, Hunter GC, et al. Correlation of ophthalmic findings with carotid artery stenosis. J Cardiovasc Surg (Torino). 1992 Jul-Aug;33(4):401-6.
5. Lawrence PF, Oderich GS. Ophthalmologic findings as predictors of carotid artery disease. Vasc Endovascular Surg. 2002 Nov-Dec;36(6):415-24.
6. McCullough HK, Reinert CG, Hynan LS, et al. Ocular findings as predictors of carotid artery occlusive disease: is carotid imaging justified? J Vasc Surg. 2004 Aug;40(2):279-86.
7. Klijn C, Kappelle LJ, van Schooneveld MJ, et al. Venous stasis retinopathy in symptomatic carotid artery occlusion: prevalence, cause, and outcome. Stroke. 2002 Mar;33(3):695-701.
8. Sivalingam A, Brown GC, Magargal LE, Menduke H. The ocular ischemic syndrome. II. Mortality and systemic morbidity. Int Ophthalmol. 1989 May;13(3):187-91.
9. Atebara N, Brown GC. Chapter 12: Ocular ischemic syndrome. In: Duane’s clinical ophthalmology [book on CD-ROM]. Vol 3. Philadelphia: Lippincott Williams and Wilkins Publishers; 2006.
10. Brown GC, Magargal LE. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol. 1988 Feb;11(4):239-51.
11. Hayreh SS, Podhajsky P, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009 Oct;116(10):1928-36.
12. Klein R, Klein BE, Jensen SC, et al. Retinal emboli and stroke: the Beaver Dam Eye Study. Arch Ophthalmol. 1999 Aug; 117(8):1063-8.
13. Arruga J, Sanders MD. Ophthalmologic findings in 70 patients with evidence of retinal embolism. Ophthalmology.1982 Dec; 89(12):1336-47.
14. Hollenhorst RW. Vascular status of patients who have cholesterol emboli in the retina. Am J Ophthalmol. 1966 May;61(5 Pt 2):1159-65.
15. Wang JJ, Cugati S, Knudtson MD, et al. Retinal arteriolar emboli and long-term mortality: pooled data analysis from two older populations. Stroke. 2006 Jul;37(7):1833-6.
16. Chang YS, Jan RL, Weng SF, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol. 2012 Oct;154(4):645-52.
17. Cooper LS, Wong TY, Klein R, et al. Retinal microvascular abnormalities and MRI-defined subclinical cerebral infarction: the Atherosclerosis Risk in Communities Study. Stroke. 2006 Jan;37(1):82-6.
18. Wong TY, Klein R, Couper DJ, et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet. 2001 Oct 6;358(9288):1134-40.
19. Panton RW, Goldberg MF, Farber MD. Retinal arterial macroaneurysms: risk factors and natural history. Br J Ophthalmol. 1990 Oct;74(10):595-600.
20. Cheung N, Rogers S, Couper DJ, et al. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke. 2007 Feb;38(2):398-401.
21. Klein R, Klein BE, Moss SE. Epidemiology of proliferative diabetic retinopathy. Diabetes Care. 1992 Dec;15(12):1875-91.
22. Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol. 1999 Nov;117(11):1487-95.
23. Duker JS, Brown CG, Bosley TM, et al. Asymmetric proliferative diabetic retinopathy and carotid artery disease. Ophthalmology. 1990 Jul;97(7):869-74.
24. Valone JA Jr, McMeel JW, Franks EP. Unilateral proliferative diabetic retinopathy. II. Clinical course. Arch Ophthalmol.1981 Aug;99(8):1357-61.
25. Gay AJ, Rosenbaum AL. Retinal artery pressure in asymmetric diabetic retinopathy. Arch Ophthalmol. 1966 Jun;75(6):758-62.
26. Dogru M, Inoue M, Nakamura M, Yamamoto M. Modifying factors related to asymmetric diabetic retinopathy. Eye (Lond). 1998;12(Pt 6):929-33.
27. Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischaemic attack in the Oxfordshire Community Stroke Project. Stroke. 1990 Jun;21(6):848-53.
28. Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007 Oct 20;370(9596):1432-42.
29. Johnston SC, Fayad PB, Gorelick PB, et al. Prevalence and knowledge of transient ischemic attack among US adults. Neurology. 2003 May 13;60(9):1429-34.
30. Giles MF, Flossman E, Rothwell PM. Patient behavior immediately after transient ischemic attack according to clinical characteristics, perception of the event, and predicted risk of stroke. Stroke. 2006 May;37(5):1254-60.
31. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischemic attack. Lancet. 2005 Jul 2-8;366(9479):29-36.
32. Tsivgoulis G, Spengos K, Manta P, et al. Validation of the ABCD score in identifying individuals at high early risk of stroke after transient ischemic attack: a hospital-based case series study. Stroke. 2006 Dec;37(12):2892-7.
33. Tsivgoulis G, Stamboulis E, Sharma VK, et al. Multicenter external validation of the ABCD2 score in triaging TIA patients. Neurology. 2010 Apr 27;74(17):1351-7.
34. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischemic attack. Lancet. 2007 Jan 27;369(9558): 283-92.
35. Chandratheva A, Geraghty OC, Luengo-Fernandez R, et al. ABCD2 score predicts severity rather than risk of early recurrent events after transient ischemic attack. Stroke. 2010 May; 41(5):851-6.

