 A 34-year-old black female presented with symptoms of bilateral redness of seven days duration. She also complained of mild pain and photophobia in each eye. The onset was gradual, and the patient reported that the condition was slowly and steadily worsening. She said that she has had similar episodes in the past.
A 34-year-old black female presented with symptoms of bilateral redness of seven days duration. She also complained of mild pain and photophobia in each eye. The onset was gradual, and the patient reported that the condition was slowly and steadily worsening. She said that she has had similar episodes in the past.
Her visual acuity was mildly affected in each eye. Biomicroscopy revealed grade 2 cells and grade 1 flare in the anterior chamber of each eye. Anterior segment examination showed the presence of mutton-fat deposits on the corneal endothelium of each eye. Iris nodules were present, and there were areas of posterior synechiae in each eye. IOP measured 9mm Hg O.D. and 10mm Hg O.S. Dilated funduscopy showed snowbanking at the inferior pars plana of each eye.
This patient had a bilateral anterior uveitis of a chronic nature, with exacerbations and remissions. There was also evidence of prior posterior segment inflammation in each eye. The mutton-fat keratic precipitates, iris nodules, posterior synechiae and posterior segment signs suggest a granulomatous etiology and a heightened likelihood of underlying systemic disease.
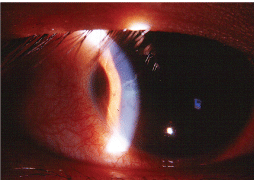
An iris nodule was present in the anterior chamber of each eye (right eye shown).
We successfully treated the anterior uveitis using topical anti-inflammatory and cycloplegic medications. However, angiotensin converting enzyme (ACE) levels were significantly elevated, and chest imaging showed bilateral hilar lymphadenopathy.
We referred the patient to a pulmonologist, who diagnosed sarcoidosis and prescribed a course of oral prednisone. The patient had a favorable ocular and systemic response to medical therapy.
Granulomatous Disease
Sarcoidosis (also called sarcoid) is a granulomatous disease that can affect virtually every body system, especially the respiratory and lymphatic systems.1 Pulmonary symptoms occur in up to one-half of all sarcoidosis patients and most commonly include dyspnea, dry cough and chest tightness or pain.1,2
When sarcoidosis affects the pulmonary system, the lungs are involved in more than 90% of patients.1 Parenchymal infiltration and irreversible pulmonary fibrosis can occur if the disease progresses, eventually leading to respiratory failure.
Sarcoidosis also can affect the lymphatic, ocular, nervous, hepatic, renal, endocrine, musculoskeletal and myocardial systems.1,2
In the
In the
Agents Implicated in the Etiology of Sarcoidosis Herpes simplex Hepatitis C Epstein-Barr Cytomegalovirus Coxsackievirus Rubella Mycobacteria (Mycobacterium tuberculosis, Mycoplasma species, atypical mycobacteria) Corynebacteria species Spirochetes Propionibacterium acnes Borrelia burgdorferi Histoplasma species Cryptococcus species Sporotrichosis. Metals (e.g., zirconium, aluminum, beryllium) Organic dusts (e.g., pine, pollen)
The precise etiology of sarcoidosis is unknown.1,2,4 The current hypothesis: In genetically susceptible individuals, sarcoidosis results from an alteration in the immune response after exposure to an environmental, occupational or infectious agent.4 Studies indicate that increased B-cell activity with elevated levels of gamma globulin (hypergammaglobulinemia) is noted in about 50% of patients. (See Agents Implicated in the Etiology of Sarcoidosis, right.)
Viruses4
Infectious agents4
Environmental antigens1,2,4
Immune dysregulation leads to a chronic T-cell (of the Th1 subtype) response, resulting in the formation of a discrete, noncaseating, epithelioid granulomas of any organ system.
The most common allele found in sarcoidosis is HLA-B8. Other alleles found include HLA-A1 and HLA-DR3.2,4
Pathways to Diagnosis
The lack of a single diagnostic test, as well as the diverse presentations, make the diagnosis challenging. Patients with sarcoidosis most commonly present in winter and early spring, suggesting an environmental trigger.2,4
For a definitive diagnosis, histologic proof of a noncaseating epithelioid granuloma, clinicoradiologic features and exclusion of similar diseases are necessary.1,5,6 A complete blood count (CBC) with differential and platelets, along with serum calcium and 24-hour urine calcium levels, should be performed if you suspect sarcoidosis.5 Serum ACE levels should be tested to help monitor both disease activity and treatment response.2,5
Because 90% of sarcoidosis patients have radiographic involvement, a postero-anterior chest radiograph should be obtained to stage the disease. The four stages:
Stage I pulmonary sarcoid: bilateral hilar lymphadenopathy (BHL).
Stage II: BHL plus pulmonary infiltrates.
Stage III: pulmonary infiltrates without BHL.
Stage IV: pulmonary fibrosis.
Pulmonary function testing can be ordered to identify defects in lung diffusing capacity and vital capacity.2,5,6
Biopsy is required for diagnosis in most cases. The skin is the most easily accessible tissue for biopsy, although conjunctival tissue has also been used. A transbronchial lung biopsy is the most accurate method and will show the presence of noncaseating granulomas.1,2,5
Treatment Overview
Most cases of sarcoidosis are not fatal, so the primary goals of treatment are to alleviate symptoms, resolve inflammatory lesions that interfere with organ function, and prevent pulmonary fibrosis. There is no curative treatment.1,2,6-8 Oral corticosteroids for a minimum of 12 months are the mainstay of treatment for pulmonary sarcoid.
Patients with Stage I disease do not require treatment.1,9 Symptomatic patients with Stage II or III disease usually show improved chest radiograph findings during treatment. Patients with Stage IV disease usually respond poorly or not at all to systemic therapy; lung transplantation may be a viable option for these patients.9
Multiple relapses may occur, and patients may require long-term, low-dose corticosteroid therapy.9 Cytotoxic agents and immunomodulators may be used in patients who do not respond to corticosteroids or as steroid-sparing agents. Rheumatrex (methotrexate, STADS Pharmaceuticals) and Imuran (azathioprine, GlaxoSmithKline) are preferred. Antimalarials such as Plaquenil (hydroxychloroquine, Sanofi Aventis) and Aralen (chloroquine, Sanofi Aventis) can also be considered in patients with pulmonary sarcoid.1,6,9,10
Sarcoidosis is an enigmatic disease that may affect all body systems. The ocular manifestations of this condition are well documented.
Consider referring any patient who presents with evidence of bilateral, granulomatous, recurrent or chronic uveitis for systemic and radiologic testing to rule out sarcoidosis. Optometrists can facilitate early diagnosis and treatment of this disease to enhance the patients quality of life.
1.Wu JJ, Schiff KR. Sarcoidosis. Am Fam Physician 2004 Jul 15;70(2):312-22.
2. Gould KP, Callen JP. Sarcoidosis. Available at: www.emedicine.com/DERM/topic381.htm (Accessed February 18, 2009).
3. Rybicki BA, Major M, Popovich J Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997 Feb 1;145(3):234-41.
4. Rossman MD, Kreider ME. Lesson learned from ACCESS (A Case Controlled Etiologic Study of Sarcoidosis). Proc Am Thorac Soc 2007 Aug 15;4(5):453-6.
5. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest 2003 Feb;123(2):406-12.
6. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest 2002 Jan;121(1):24-31.
7. Martin WJ 2nd, Iannuzzi MC, Gail DB, Peavy HH. Future directions in sarcoidosis research: summary of an NHLBI working group. Am J Respir Crit Care Med 2004 Sep 1; 170(5):567-71.
8. Grutters JC, van den Bosch JM. Corticosteroid treatment in sarcoidosis. Eur Respir J 2006 Sep;28(3):627-36.
9. Nunes H, Bouvry D, Soler P, Valeyre D. Sarcoidosis. Orphanet J Rare Dis 2007 Nov 19;2:46.
10. Hilton JM, Cooper DM, Henry RL. Hydoxychloroquine therapy of diffuse pulmonary sarcoidosis in two Australian male children. Respirology 1997 Mar;2(1):71-4.

