Few would argue that optical coherence tomography (OCT) technology has altered the landscape of eye care for monitoring and understanding ocular pathology. Time-domain OCT has been described as revolutionary, Fourier-domain OCT as evolutionary, but what about anterior-segment OCT? The often-overlooked ugly duckling of the OCT applications, AS-OCT has the potential to grow into a showstopper in optometric care.
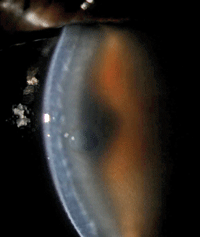
1. Note the dehiscence of the graft and substantial edema in this image, taken one week after DSAEK surgery.
Perhaps AS-OCT is a little less glamorous than the retinal and retinal nerve fiber layer scanning modules; it certainly is less frequently discussed. Many clinicians feel they could gather the same data with AS-OCT that they can already acquire with ultrasound biomicroscopy (UBM) or, to a lesser degree, Scheimpflug-based imaging. However, as OCT technology has evolved toward quicker, higher-sensitivity, higher-quality Fourier domain, it has achieved lateral (15µ) and axial (5µ) resolution superior to these other techniques.1 While UBM allows for imaging through opaque media—a claim anterior-segment OCT cannot make—the OCT is simpler to use, requiring less staff training and patient preparation.
Yet, I’ll be the first to admit that despite having access to AS-OCT, I’m often guilty of underusing it—and I get the sense that I’m far from the only one. In the cases that follow, we’ll look at how AS-OCT can be in useful in providing better patient education, setting reasonable surgical expectations with patients, and delivering data that helps optometrists and surgeons to make more informed decisions regarding surgical eligibility and the next steps in planning. Given its clinical utility, we may soon see the use of AS-OCT growing at a more rapid pace than its popular posterior counterpart.
Case 1: Patient Education
An 87-year-old white female presented at her one-day postoperative exam following Descemet’s stripping automated endothelial keratoplasty (DSAEK). She had hand motion vision in the treated eye, the cornea had 3 to 4+ epithelial and stromal edema, and the graft was very thickened but appeared to be apposed to the host stroma. A 20% air bubble remained in the superior anterior chamber.
After seeing the patient that day, the surgeon was quite convinced that, even though the positioning looked acceptable, it was unlikely the graft would clear. So he scheduled a re-graft for the following week—to be cancelled if the graft and host began to deturgesce.
2. Dehisced posterior graft with significant corneal edema and a central bulla.
One week later, the patient’s vision remained poor and the graft had fallen out of position centrally (figure 1). Edema remained and a central bulla had developed (figure 2). AS-OCT showed remarkably thickened donor and host tissue as well as an obvious dehiscence of the graft.
Prior to re-graft, we reviewed the AS-OCT with the patient and her family, which helped them to better understand the problem we needed to correct and reduced their level of anxiety with the surgical plan. We diagnosed the case as a failed DSAEK graft. The patient underwent re-graft as planned and did well, with acuity measuring 20/100 at one day and 20/50 at one week.
Case 2: Screening/Decision Making


3. Diffuse, hazy anterior stromal appearance of the affected eye.
4. Iris laceration.
A 55-year-old white female presented with a glass injury to her eye sustained in a motor vehicle accident 30 years prior.
Her new primary OD had referred her to our office for an evaluation for phototherapeutic keratectomy (PTK).
Over the years, she had a series of procedures to remove the embedded glass from her eye—in the anterior corneal stroma primarily, though there was one area of full-thickness penetration and intraocular damage that manifested as an iris tear and localized cataract (figures 3 and 4).
As a result, she developed an unusually diffuse reticular scar to her cornea. Since the time of her accident, the patient’s vision had been relatively stable, best corrected to 20/70 levels (per her report) and her prescription was a stable +2.50-1.25x014.
Her keratometric data was only mildly irregular and a rigid gas permeable contact lens over-refraction had no impact on acuity (figure 5). Slit-lamp findings were unremarkable other than the previously described abnormalities.
We diagnosed the patient with anterior stromal scarring secondary to ocular trauma.


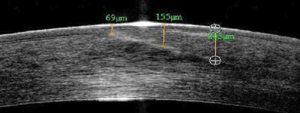
6. Plane of irregularity
appeared uniform and superficial—note the hyper-reflective zone in the
anterior stroma and the full-thickness irregularity to the left edge of
the scan.
5. Relatively normal keratometric topography.
Deep anterior lamellar keratoplasty (DALK) was not possible given the single area of corneal penetration, so we considered three options—PTK, penetrating keratoplasty (PK) or no treatment.
AS-OCT helped us determine the best course of action.
After measuring the depths of central scarring with AS-OCT, we found the patient to be a good candidate for PTK treatment and decided to proceed with the surgery.
Standard PTK was performed with a paired anti-hyperopic treatment to reduce postoperative anisometropia. One week after surgery, the patient saw 20/30 uncorrected and noticed a substantial improvement in her vision.
The scarring was essentially eliminated with the exception of the full-thickness zone, and the patient was very pleased with the outcome (figure 6). She returned to the care of her primary eye doctor.
Case 3: Scar Runs Deep
A 57-year-old white male came to our clinic about a month after sustaining a corneal injury when he was hit in the right eye by a tree branch at work, presumably while operating a piece of heavy machinery. An outside optometrist had provided good care to the patient during the acute period, and referred him to our practice for a surgical evaluation after the eye stabilized.
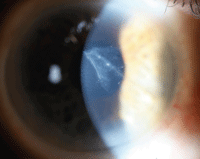
7. Slit-lamp exam showed a triangular central and anterior scar, with a tail trailing deeper into the cornea to its terminal point about 1.5mm from the nasal limbus, where it appeared to penetrate the endothelium.

8. Irregular corneal topography: paracentrally steep island corresponding to the primary body of the scar.
Best spectacle-corrected acuity was 20/50, and an over-refraction with a gas permeable lens minimally improved his vision to 20/40. Following his injury, the patient was very symptomatic of aberrations, glare and halos and had significant reported disability, paired with the right eye being his strongly dominant eye.
Slit-lamp examination showed a triangular central and anterior scar, with a tail trailing deeper into the cornea to its terminal point about 1.5mm from the nasal limbus where it appeared to penetrate the endothelium (figure 7).
There were no signs of deeper disruption to the eye, and the primary optometrist had indicated that the patient never developed traumatic retinopathy. Topographic measures showed a paracentrally steep island corresponding to the primary body of the scar (figure 8).
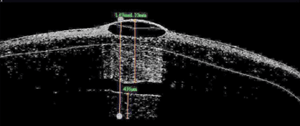
AS-OCT revealed a sloped disruption to the cornea that dove from roughly 70µm in depth at the temporal edge of the scar to 243µm within the nasal margin of the visual axis. An endothelial disruption was noted on the nasal edge of the scar (figures 9 and 10). We diagnosed the presentation as a traumatic corneal injury with subsequent full-thickness scar.
We informed the patient that because of the depth of the scar, he was not an ideal candidate for PTK. We further explained that while PK or DALK would be curative, either would require several months of follow-up and he would likely still require a contact lens for best vision. Observation seemed to be the best option, as long as the patient was improving.
As he stabilized over the next six months, he became more vocal about his desire to do something. He didn’t want to go the route of transplantation, and said he would be satisfied with an improvement in his symptoms.
After significant consultation with the patient and our surgeon, we decided to attempt PTK—knowing that a large portion of the scar would remain below treatable depths, but that some of the central opacity could be removed and paired with flattening of the steep and irregular paracentral topography.
The prognosis for improvement was good, but was poor for symptom resolution. The patient understood and elected to proceed with scheduling, for which we are awaiting insurance approval.
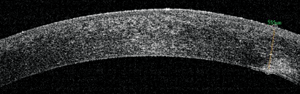
9. AS-OCT image of the grade of the penetrating tunnel.
10. AS-OCT showing a penetrating scar of the endothelium.
Case 4: A Steep Risk
A 61-year-old white male presented to clinic for cataract evaluation OD. He had undergone LASIK surgery on both eyes 15 years prior and had done well until the last 18 months, when he noted a significant decrease in his vision OD. He had LASIK in Alaska and his chart had been destroyed, so no preoperative data was available. He did, however, provide the history that he was significantly nearsighted and considered himself “legally blind without glasses.”
He was correctable to 20/30 and had glare-reduced acuity at 20/400. The eye had developed 2D of myopia over the last four years, according to his current primary optometrist’s notes. Evaluation showed normal ocular structures with the exception of old, nearly invisible LASIK flaps OU and, in the left eye only, a mix of cortical and anterior subcaspsular changes in the lens.
We obtained variable measures of his corneal power including standard topography with Vista (Eyesys Technology), Scheimpflug-based topography with Pentacam (Oculus), automated keratometric measures with IOL Master (Carl Zeiss), and corneal power with AS-OCT using RT-Vue (Optovue).
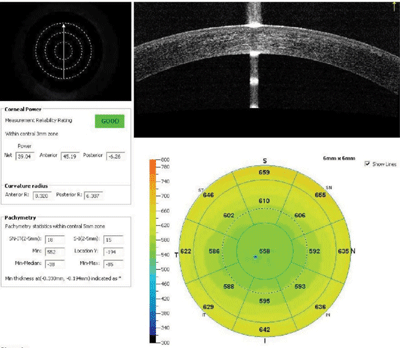
11. Various measures of this patient’s corneal power included standard topography, Scheimpflug-based topography, automated keratometry and corneal power with anterior-segment OCT.
The two measures of total corneal power accounting for anterior and posterior curvatures from Pentacam and RT-Vue showed good agreement (figure 11).
IOL Master automated keratometry and traditional topography also matched up; however, at 41.00D, these were nearly 2.00D steeper than the powers estimated by AS-OCT and Scheimpflug, which were at roughly 39.00D. Further, there was moderate irregularity in the central 3mm zone.
Based on the corneal irregularity and agreement between the RT-Vue and Pentacam, we decided to shift keratometric input toward the AS-OCT/Scheimpflug value. We educated the patient about the inherent difficulty in predicting his outcome, and discussed the possibility of an IOL exchange, should it be necessary. We also reviewed the normal risks of cataract surgery, and the patient elected to proceed as scheduled.
At his one-day postoperative exam, his uncorrected vision was 20/20 and he was very pleased with his results. We sent the patient back to his primary OD for care. In this case, AS-OCT provided us with a level of specificity and accuracy that ensured the best outcome. If standard keratometric results had been used, he would have ended up approximately 1.50D hyperopic.
Discussion
In the surgical management of corneal pathology, the primary question that needs to be answered in many cases is: Is there a less invasive option than PK for this patient?
The historic limitations of PK are well known—including lifetime risk of rejection, glaucoma, infection, high dependence on gas permeable contact lenses—and highlighted by the lifetime follow-up care needed to ensure both refractive function and viability of the graft.
While DALK presents an option with reduced lifetime rejection and endothelial failure issues, it still requires a significant time investment for visual rehabilitation and a high reliance on gas permeable contact lenses for best vision.
PTK, however, eliminates both the need for long-term refractive rehabilitation and the lifetime effects of simply having a graft placed on the eye. However, while the acceptable depth of treatment with PTK is deeper than what is achievable with surface treatments like superficial keratectomy, it remains limited, with “acceptable” varying based on the study, but ranging from 20% to 50%.2,3 Therefore, determining the depth of corneal pathology accurately with anterior-segment OCT allows for a more realistic prognosis and better patient selection.
Moving from PTK to transplantation, there can occasionally be clinical difficulty in determining whether a patient’s pathology affects the posterior stroma alone or if the endothelium has also been compromised. In cases of corneal trauma, where only the posterior stroma has been compromised, DALK would be the procedure of choice for surgeons who have added this difficult technique to their repertoire.
However, the most frequently encountered intraoperative complication of DALK is perforation of the endothelium—a complication that may require transition to a full PK and is more likely in the case of endothelial involvement.4 In cases where there is question of endothelial compromise, an AS-OCT can help to clearly define whether the patient would fare better with DALK vs. PK—or at the very least, give the surgeon a reasonable idea that the case will be more or less likely to require transition to PK mid-surgery.
A rapidly developing area of interest in surgical eye care is refining techniques to more accurately predict corneal power after refractive surgery prior to cataract surgery. It’s widely known that prior refractive surgery disrupts the accuracy of measures of true corneal power, a metric required for appropriate IOL calculations.
This disruption is at least partially based on the fact that corneal power calculations use a thin-lens model of the cornea, despite its having distinct anterior and posterior curvatures.5
To account for this problem, and allow only anterior curvatures to be gathered, formulas for calculation have relied on a known “normal” ratio of anterior to posterior corneal curvature to give an overall corneal power. Refractive surgery disrupts this ratio by changing anterior curvature without having a significant effect on posterior curvature—a trend that is becoming more common with the continued increase in refractive treatment.
Therefore, it could be expected that devices that allow capture of both anterior and posterior corneal curvatures may perform superiorly in lens calculations for patients with previous refractive surgery. AS-OCT has this ability, and the device is widely available in many surgery centers.
Bear in mind that the results described in Case 4 are not universal, and I have had experiences of the traditional keratometric data performing as well as, or better than, AS-OCT for post-refractive surgery patients. Ability to plug in the data readily seems to vary from case to case. These results are not developed to be inserted into standard IOL power calculation formula, like the Holladay 2, Hoffer-Q and SRK/T formulas, and so applying it in this manner is an off-label use of both the formula and the data. However, having the data to compare directly against more standard keratometric measures for agreement can give the clinician an idea of the validity and accuracy of the traditional measurement.
Also, the ability of this device to estimate corneal power—perhaps more accurately than traditional measures—creates an intriguing inroad for developing more accurate lens calculation formula. As more post-refractive patients begin making their way into our cataract clinics along with their high refractive expectations, AS-OCT’s ability to measure corneal power may prove to be as valuable and just as widely used as the posterior segment module.
While AS-OCT and retinal OCT share much of the same technology, clinicians must remember they are essentially different devices with distinct applications. Whereas use of retinal OCT is typically for diagnosis and monitoring response to treatment, AS-OCT is generally less useful in diagnosis of pathology but better suited for treatment decision-making. However, AS-OCT does provide valuable objective information that aids in the development of an effective treatment paradigm for anterior segment disease—and like the retinal OCT, gives a clear, easily readable image for improved interpretation and patient education.
Dr. Bronner is a staff optometrist at the Pacific Cataract and Laser Institute in Kennewick, Wash. He has no financial interest in any of the products mentioned.
1. Ventura BV, Moraes HV Jr, Kara-Junior N, Santhiago MR. Role of optical coherence tomography on corneal surface laser ablation. J Ophthalmol. 2012;2012:676740. Epub 2012 Sep 5.
2. Rashad MA. Pentacam-based phototherapeutic keratectomy outcome in superficial corneal opacities. Clin Ophthalmol. 2012;6:885-94. Epub 2012 Jun 14.
3. Faktorovich EG, Badawi DY, Maloney RK, Arivasu RG. Growth factor expression in corneal wound healing after excimer laser keratectomy. Cornea. 1999 Sep;18(5):580-8.
4. Reinhart WJ, Musch DC, Jacobs DS, et al. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty, a report by the American Academy of Ophthalmology. Ophthalmology. 2011 Jan;118(1):209-18.
5. Holladay JT, Hill WE, Steinmueller A. Corneal power measurements using scheimpflug imaging in eyes with prior corneal refractive surgery. J Refract Surg. 2009 Oct;25(10):862-8.

