Until recently, the choroid’s inaccessibility—essentially buried beneath the retina—has made it a little understood anatomical structure. But this thin layer between the sclera and the retina, the “middle coat” of the eye, is vital to ocular health. It supports the outer layers of the retina by supplying nutrients and removing waste. Choroidal melanocytes absorb intraocular scattered photons (light). The superfluous flow of blood through the choroid assists in the removal of heat derived from the metabolism of phototransduction. Also, the suprachoroid lamina provides a safe route of travel for the long posterior ciliary arteries and nerves as they course toward the anterior aspect of the globe.
Choroidal analysis, in addition to retinal imaging, can provide supplemental information regarding disease progression. Thinning of the choroid is an indicator of advancing stages of nonexudative age-related macular degeneration (AMD) and correlates with the rate of visual field loss in eyes with normal tension glaucoma (NTG).1,2
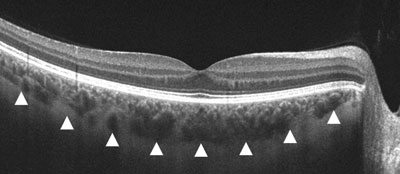 | |
| Gray-scale EDI-OCT of a healthy eye. The white arrows correspond to the choroid/scleral junction. |
Today, we’re able to image deeper into the eye than ever before, allowing us the opportunity to evaluate choroidal thickness and morphology both for the benefit of patient treatment and for a better understanding of retinal diseases.
The technology making this possible is enhanced depth choroidal imaging, a function of optical coherence tomography (EDI-OCT). The clinical uses of EDI-OCT include detection and monitoring of pathologic alterations in choroidal thickness, differentiation between diseases with similar clinical features, and precise choroidal measurements. As this technology finds its way into our exam lanes, we can take on a greater role in patient management, one previously in the sole purview of the retina MD.
This article outlines the capabilities of EDI-OCT and the research that’s helped us get to this level of care.
The Development of EDI
EDI was pioneered by ophthalmologists Ron Margolis, MD, and Richard F. Spaide, MD, in 2009.3 Before their work, OCT imaging of the choroid was virtually impossible because of poor light source penetration through the densely pigmented retinal pigment epithelium, light scatter by the choroidal vasculature itself, limited axial resolution and motion artifacts.3 Using the Spectralis (Heidelberg Engineering), Drs. Margolis and Spaide found that they could more effectively view the choroid by inverting the image.3,4 Originally, they accomplished this by simply positioning the patient slightly closer to the machine.3,4 By convention, the zero delay line is located at the top of the imaging screen and represents the area of most precise focus.3,5 By inverting that image, the choroid/sclera interface is placed closer to that zero delay line, improving the scan quality of the deeper, posterior structures.3 EDI-OCT penetrates an additional 500µm to 800µm deeper compared with traditional OCT imaging.6
| Other Choroid Imaging Modalities In addition to OCT-EDI, swept-source OCT (SS-OCT) and image averaging OCT techniques are used to improve visualization of the choroid.1 SS-OCT imaging uses a variable wavelength, frequency-swept laser light source.1,2 Adding a longer-wavelength source allows even deeper tissue penetration without sacrificing the resolution of vitreoretinal structures that are better imaged at shorter wavelengths.1 Another advantage of SS-OCT imaging is that interference patterns are more efficiently detected by photodiodes as opposed to conventional spectrometers.1,2 The result is an axial resolution of 5.3µm with an acquisition speed of 100,000 to 400,000 A-scans per second.3 Image averaging refers to the technique of overlying multiple B-scan images of the same retinal location.2 Since noise is random, combining many scans effectively cancels out the “speckle” or “static” present in each image.2,4 This increases the signal-to-noise ratio, which sharpens and enhances retinal and choroidal features.2 The quality of image averaging is improved with either eye tracking (Spectralis, Heidelberg Engineering) or Selective Pixel Profiling software capable of evaluating all of the pixel data to construct the best possible image (Cirrus HD-OCT, Carl Zeiss Meditec).4-7 When using the Cirrus HD-OCT, image averaging is maximized by combining the five lines of the HD 5-line Raster scan into one line (0mm spacing).7 1. Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013 Sep-Oct;58(5):387-429. 2. Adhi M, Duker JS. Optical coherence tomography: current and future applications. Curr Opin Ophthalmol. 2013 May;24(3):213-21. 3. Raiji V, Walsh A, Sadda S. Future directions in retinal optical coherence tomography: a review of new technologies on the horizon. Retinal Physician. Available at: www.retinalphysician.com/articleviewer.aspx?articleID=107030 (accessed March 14, 2014). 4. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008 Oct;146(4):496-500. 5. Lin P, Mettu PS, Pomerleau DL, et al. Image Inversion Spectral-Domain Optical Coherence Tomography Optimizes Choroidal Thickness and Detail through Improved Contrast. Invest Ophthalmol Vis Sci. 2012 Apr 6;53(4):1874-82. 6. Puliafito C. Cirrus HD-OCT today and tomorrow: upcoming analysis capabilities complement unsurpassed image quality and ease of use. Ophthalmology Management. Available at: www.ophthalmologymanagement.com/articleviewer.aspx?articleID=104083. (accessed March 14, 2014). 7. Carl Zeiss Meditec, Inc. (2011). Cirrus HD-OCT: How to read the Cirrus reports. Available at: www.meditec.zeiss.com/88256DE3007B916B/Order?OpenForm&RetinalImaging_EN. |
EDI-OCT can now be easily performed with just the click of a button on most OCT models that have new software upgrades, including the Spectralis, Cirrus HD-OCT (Carl Zeiss Meditec), and RTVue (Optovue).6 The highest quality image of the choroid that can be obtained using commercially available machines is accomplished by combining EDI-OCT with image averaging.6 When using the Cirrus HD-OCT, this involves combining the five lines of the HD 5-line Raster scan into one line (0mm spacing).7
Normal Choroidal Morphology
The thickness of the choroid varies throughout the posterior pole. In healthy eyes, the choroid is typically thickest beneath the fovea, where its average thickness ranges from 262µm to 354µm.3,8-10 The surrounding superior and inferior choroidal quadrants within the macular region are generally thicker than the nasal and temporal quadrants.10 The temporal choroid is thicker than the nasal choroid, which decreases rapidly toward the optic nerve.3,8,10,11 Generally, the superior choroid is thicker than the inferior choroid.10 This thinning of the inferonasal macular choroid marks the location of the embryonic optic fissure and may represent a normal “relative coloboma” or “knitting area.”10,12-14 Additionally, the thinning of the choroid between the fovea and the optic nerve may predispose the formation of peripapillary atrophy.11 In the optic nerve, the peripapillary choroid is thinnest inferiorly and may contribute to the pathogenesis of glaucoma in susceptible cases.4,11,12
Influencing Choroidal Health
Many factors influence choroidal thickness—most significantly, age.10 In patients older than about 60, choroidal thickness progressively decreases by 4µm to 5µm each year.8,15 Although great variation exists between individuals, the resultant mean subfoveal thickness in individuals older than age 60 is 197µm.8,10 Age has little effect on choroidal thickness in younger patients.5 This age-related thinning of the choroid is likely mirrored by a reduction in oxygen and metabolite supply to the retinal pigment epithelium (RPE) and outer retina, which may play a role in the development of AMD and other degenerative retinal conditions.3,8,11 Studies have already demonstrated a correlation between macular choroidal thickness and best-corrected visual acuity, highlighting the functional dependence of the photoreceptors on choroidal support.1,15,16
On average, healthy males have a 7% greater choroidal volume, which may explain the greater incidence of AMD among females who have thinner choroids at the outset.11,15
Other influential factors on choroidal thickness are refractive error and axial length.10,15 Studies have demonstrated a negative correlation between choroidal thickness and degree of myopia.8,10,15 Similarly, increasing axial length is also associated with a decrease in choroidal thickness.10,11 In eyes with myopia of greater than 1.00D, subfoveal choroidal thickness is expected to decrease by approximately 15µm per diopter of myopia and 32µm for each 1mm increase in axial length.15
Other biometry measures associated with a decrease in choroidal thickness include shallower anterior chamber, thinner lens and steeper cornea.15 Interestingly, neither hyperopic refractive error nor retinal thickness correlate well with choroidal thickness.9,12,15
It is well known that the choroid exhibits a pattern of diurnal fluctuation that may be related to fluctuations in choroidal blood flow given that the choroid is not autoregulated.17,18 Investigators measured choroidal thickness over a 24-hour period and found that the choroid was generally thicker between 3 a.m. and 9 a.m. and thinnest between the hours of 3 p.m. and 9 p.m.18
A change in posture at night likely causes a gravitational elevation in venous hydrostatic pressure, resulting in engorgement of choroidal veins and an increase in choroidal blood volume.19 No correlation between intraocular pressure (IOP) and choroidal thickness has been authenticated.18
Outer Retinal Diseases
• AMD. The invaluable functions of the choroid (waste removal from the RPE and photoreceptor nutrient supply) suggest that choroidal dysfunction is likely involved in the pathogenesis of AMD.20,21 For instance, a 2011 study reported a decrease in mean subfoveal choroidal thickness in patients with exudative AMD (195µm) and nonexudative AMD (213µm) compared with age-matched healthy eyes (272µm).22
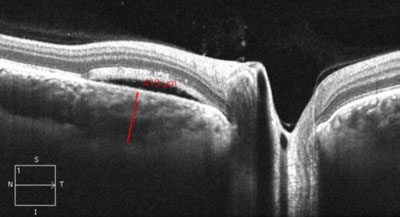 | |
| EDI-OCT showing central serous chorioretinopathy and neurosensory retinal detachment demonstrating an increase in nasal peripapillary choroid thickness (413µm). |
Similarly, a 2013 study of choroidal thickness in eyes with nonexudative AMD found that worsening of the disease is associated with a progressive decrease in subfoveal choroidal thickness.1 As expected, this study also reported a negative correlation between the subfoveal choroidal thickness and visual acuity, highlighting the importance of choroid health and visual function.1
Additional research measured choroidal thickness in eyes with early AMD either with or without reticular pseudodrusen, an interlacing yellowish pattern often found in the superior perifovea in some AMD patients.23,24 The authors reported that the presence of reticular pseudodrusen is associated with a decrease in subfoveal choroidal thickness by approximately 19%.23 This suggests that both the presence of reticular pseudodrusen and decreased choroidal thickness may be risk factors for progression to advanced AMD.23,24
Using laser Doppler flowmetry, new studies demonstrate a decrease in foveolar choroidal blood flow in eyes with nonexudative AMD correlating directly with the severity of the disease.21,26,27 In fact, according to one study, decreased choroidal blood flow precedes conversion to exudative AMD and vision loss.27 This decrease in choroidal blood flow and volume may account for the decrease in choroidal thickness found using EDI-OCT.21 These findings may indicate the need for serial measurements in newly discovered and advancing cases.
Analysis of choroidal thickness not only plays a role in assessing the risk of developing AMD and AMD progression, but may also aid in differentiating between AMD and other diseases of choroidal circulation such as central serous chorioretinopathy (CSCR), polypoidal choroidal vasculopathy (PCV) and adult-onset foveomacular vitelliform dystrophy with fluid accumulation.28-30 EDI of the choroid has increased our understanding of the pathogenesis of AMD and, with the advent of automated OCT choroidal thickness measurement, has the potential to reduce the time and labor costs associated with AMD management.31,32
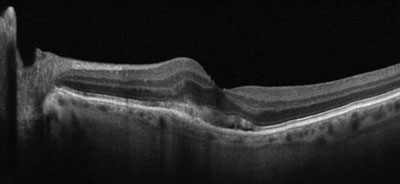 | |
| Gray-scale EDI OCT of an eye with exudative AMD and a perifoveal choroidal neovascular membrane with overlying subretinal fluid. Note the severe thinning of the subfoveal and nasal choroid. |
• Central serous chorioretinopathy. CSCR is caused by increased choroidal hyperpermeability and breakdown of the outer blood retinal barrier, which leads to exudative/serous detachment of the neurosensory retina from the RPE.33 Several studies using EDI-OCT documented significant increases in choroidal thickness in eyes affected with acute CSCR.34-36 One study found a 125µm increase in mean subfoveal choroidal thickness in eyes with CSCR compared with age-matched controls.35 These findings are also supported in principle by investigators who found an 85% increase in choroidal thickness compared with age-matched controls.35 This increase in choroidal thickness suggests that elevated hydrostatic pressure within the choroid may be a key pathophysiological factor in CSCR.37
In cases of chronic CSCR, treatments of laser photocoagulation or photodynamic therapy (PDT) or both may become necessary. Indeed, a 2010 study reported a 59µm decrease in mean choroidal thickness at one month following PDT treatment in eyes affected with CSCR.38 A later study, using half-dose PDT, reported a mean decrease of 74µm at one month that was sustained to one year in 13 CSCR patients.39,40
With the use of EDI-OCT technology, choroidal thickness can be a useful parameter to assess CSCR activity both before and after treatment.
• Polypoidal choroidal vasculopathy. PCV is a primary disease of the choroid resulting in recurrent exudative and hemorrhagic pigment epithelial detachments with associated serous macular detachment.40-43 The definitive diagnosis is made by indocyanine green angiography, which reveals multifocal choroidal hyperfluorescence, dilated and hyperpermeable choroidal veins, and a branching choroidal vascular network with adjoining polypoidal vessel dilatations.40-44 Although the clinical manifestations and genetic mutations associated with PCV are similar to AMD, PCV is more common among Asian and black patients as compared with white patients.42,43,45 PCV is less aggressive than AMD and the visual prognosis is better than that of exudative AMD.40,46
Several studies have shown the subfoveal choroid is bilaterally thicker by 80µm to 210µm in patients with PCV compared with healthy controls.40,41,46 Further, the choroidal thickness decreases following treatment with PDT.47 Additionally, investigators found a positive correlation between the diameter of the largest choroidal vessel lumen and the increase in choroidal thickness.41 This suggests that thickening of the choroid in PCV is likely due to vessel dilation and engorgement rather than extravasation of fluid into the interstitial stroma via hyperpermeability.40,41
Some postulate that the dilation of large choroidal vessels may be caused by venous stasis, an abnormality in autonomic innervation to the choroid, or an increase in ocular perfusion pressure.40,41,46 Research demonstrates that the mean ocular perfusion pressure, defined as the difference between mean arterial blood pressure and venous pressure, is significantly elevated in eyes with PCV.46 These findings agree with previous research suggesting that systemic hypertension, which elevates ocular perfusion pressure, is a risk factor for PCV.46,48 Increased ocular perfusion pressure puts mechanical stress on the fragile choroidal vessels, contributing to vessel dilation and increased hydrostatic pressure within the choroidal vasculature favoring fluid flux out of the vessels and into the interstitial stroma.46,49
Evaluating subfoveal choroidal thickness using EDI-OCT technology may be useful as a method to differentiate between PCV and AMD (associated with choroidal thinning).50 In one study, eyes with a subfoveal choroidal thickness of 300µm or greater were five to six times more likely to have PCV.50 Given the discrepancy in choroidal thickness values, it is likely that AMD and PCV have diverse etiologies and that PCV is not a subtype of AMD as previously thought.40,41
In addition to an increase in choroidal thickness, EDI-OCT often shows, in cases of PCV without hemorrhage, a separation of the RPE from Bruch’s membrane referred to as the “double-layer sign.”41 The space between the RPE and Bruch’s membrane is moderately hyper-reflective and may be composed of vascular networks, polypoidal lesions, sub-RPE hemorrhage or cloudy fluid.41,51
In non-hemorrhagic cases, research shows Bruch’s membrane remains intact.41 Researchers postulate that hemorrhaging in PCV may be caused by ruptures of Bruch’s membrane.41
• Retinitis pigmentosa. Retinitis pigmentosa is a genetic disease of variable inheritance causing destruction of the outer retina and RPE.16 It classically results in night blindness and constriction of the peripheral visual field.52 Several studies employed EDI-OCT to investigate choroidal thickness in eyes with retinitis pigmentosa. The results showed a decrease in subfoveal choroidal thickness by 27% to 36% compared with unaffected controls.16,52 One group found subfoveal choroidal thickness significantly correlated with acuity and duration of the disease.16 They, and others, postulate that the decrease in choroidal thickness may be due to a reduction in choroidal blood flow velocity and volume found in eyes with retinitis pigmentosa.16,52,53
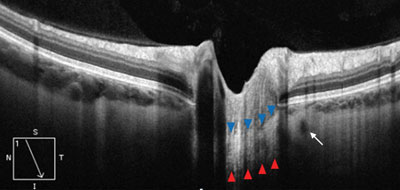 | |
| EDI-OCT of deep papillary structures in a healthy eye. The blue arrows outline the anterior border of the hyper-reflective lamina cribrosa, while the red arrows outline the posterior border. The small hyporeflective circle adjacent to the laminar optic nerve represents the vascular lumen of the circle of Zinn-Haller (white arrow). |
Inner Retinal Disease
• Diabetic retinopathy. Diabetic retinopathy is the leading cause of new cases of blindness among adults ages 20 to 74.54 Clinically, the pathophysiology of diabetic retinopathy is attributed to vascular changes within the retina. However, many histological studies identify similar signs of vascular damage within the choroid.55-58 One study describes a significant decrease in choroidal blood flow within eyes of Type 2 diabetics, particularly those with macular edema.59 EDI-OCT technology has helped to reveal decreased choroidal thickness in various stages of the disease.60,61
One study described significant thinning in patients with diabetic macular edema and in patients with treated proliferative diabetic retinopathy.60 However, no changes in choroidal thickness were observed in patients with nonproliferative diabetic retinopathy.60 Another found choroidal thinning in all stages of the disease when compared with age-matched controls.61 These findings underscore the potential for analysis of the choroid as a method of monitoring the severity and progression of retinopathy.
EDI-OCT for Glaucoma
With the advent of EDI-OCT, imaging of the peripapillary choroid as well as other deep papillary structures—such as the lamina cribrosa, short posterior ciliary arteries, central retinal artery, central retinal vein and the subarachnoid space around the optic nerve—is now possible.62
• Thinning of the lamina cribrosa. Recent evidence suggests that the primary site of ganglion cell axon injury is likely within the lamina cribrosa.62-64 The biomechanical theory of glaucoma centers around the idea that an increase in IOP in the setting of reduced cerebrospinal fluid pressure causes posterior bowing and distortion of the lamina cribrosa, leading to crush damage to the axonal fascicles that pass through it.63,64 EDI-OCT of the lamina cribrosa shows the lamina tends to be thinner in eyes with primary open angle glaucoma (POAG) compared with normal eyes.65,66 As theorized, investigators have demonstrated the lamina is thinnest, approximately half that of normal eyes, in eyes with NTG.65 Researchers suspect the unusually thin lamina cribrosa found in NTG eyes is highly susceptible to deformation, such that it occurs even in the presence of normal IOP values.65 Combined with the possibility of poor vascular perfusion, this finding reinforces the biomechanical theory of glaucoma.
Several studies have shown a correlation between laminar thinning and the degree of structural and functional glaucomatous damage.65,67 One found mean laminar thicknesses of 234µm in eyes with early stage glaucoma, 179µm in eyes with moderate stage glaucoma and 156µm in eyes with advanced stage glaucoma.65 Additionally, the lamina was significantly thinner in NTG eyes with disc hemorrhage compared with those without.65 The investigators hypothesized that a disc hemorrhage was likely a result of failure in capillary-containing laminar beams that led to rupture of prelaminar capillaries.65 Research shows a significant correlation between decreasing laminar thickness and worsening visual field mean deviation.65,67 This suggests the laminar thinning plays a role in progressive glaucomatous vision loss.65,67
Regional variation and changes in laminar pore size, as well as changes in pore shape, may be linked to glaucomatous progression too.62,68 A recent study found variations in laminar pore shape and size as well as focal areas of partial laminar tissue loss in several glaucomatous eyes when evaluated with EDI-OCT.62 In eyes with localized nerve fiber layer loss, corresponding focal laminar defects were evident.69
Until further research is completed, it remains uncertain whether thinning and other structural changes of the lamina cribrosa are causes or consequences of glaucomatous optic nerve damage.66
• Choroidal thickness in OAG and NTG. Evidence evaluating the choroidal thickness in eyes with glaucoma is conflicting. Some reports suggest choroidal thickness is less strongly correlated with the degree of glaucomatous damage compared with lamina cribrosa thickness. These reports, using EDI-OCT, show subfoveal and peripapillary choroidal thickness is not significantly different between eyes with OAG and OAG suspect eyes.70,71
To the contrary, choroidal thickness in the temporal peripapillary location (between the macula and nerve) is thinner in NTG patients than normal eyes and is correlated with the rate of visual field loss.2 These findings can be interpreted to mean that hemodynamic alterations occur in the short posterior ciliary arterial supply to the posterior choroid along with the laminar/prelaminar optic nerve head.2,72
• Choroidal thickness in angle closure. A 2003 study proposed that choroidal expansion may contribute to the pathogenesis of primary angle closure.74 The authors theorized an increase in choroidal volume would lead to an increase of posterior segment volume and pressure, inducing anterior displacement of the lens and shallowing of the anterior chamber.74,75 Mechanisms that may induce choroidal expansion include:
- An increase in episcleral and orbital venous pressure.
- An increase in the osmotic pressure of the extravascular choroidal stroma due to leakage of large proteins that favor fluid movement into the extravascular space.74
The authors believe that primary angle closure may represent choroidal expansion in already smaller, anatomically predisposed eyes.74,76
EDI-OCT studies examining the choroid in patients with variants of primary angle closure support the hypothesis of choroidal expansion. A 2013 study evaluated subfoveal choroidal thickness in four patient subgroups of angle closure: primary angle-closure suspects, acute primary angle closure, primary angle closure and primary angle-closure glaucoma. Compared with the non-glaucomatous control group, the choroid was thicker in all subtypes of angle closure.75 The mean subfoveal choroid was thickest in the acute primary angle-closure group—62µm thicker than control eyes.75 A similar study found an increase in subfoveal choroidal thickness of approximately 60µm compared with controls in the fellow eyes of patients with a history of acute primary angle closure.77
A thick choroid may be another anatomical characteristic associated with primary angle closure that increases the risk of this entity, along with shallow anterior chamber depth, shorter axial length and increased lens thickness.75
Choroidal Tumors
• Choroidal nevus and choroidal melanoma. Choroidal melanoma is the most common primary adult ocular malignancy.78 Risk factors for choroidal melanoma include orange pigment (lipofuscin), subretinal fluid, tumor thickness greater than 2mm via ultrasonography, symptoms of flashes, floaters or blurred vision and proximity to the optic nerve.79, 80
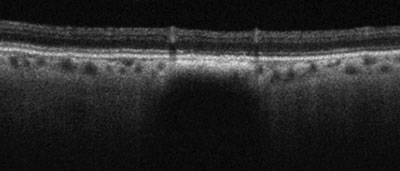 | |
| Gray-scale EDI OCT of a small choroidal nevus with attenuation of the choriocapillaris and deep posterior choroidal shadowing. Note the preservation of the overlaying retina and absence of elevation or subretinal fluid. |
EDI-OCT allows for easy measurement of choroidal tumor thickness and detection of associated retinal edema and subretinal fluid.79 Several authors have found that traditional ultrasonography tends to overestimate choroidal tumor thickness, sometimes more than two times greater than OCT measures.81-83 In 2012, investigators compared EDI-OCT with ultrasonography in the evaluation of 51 cases of choroidal nevi. The authors found that EDI-OCT features of choroidal nevi included overlying choriocapillaris thinning, RPE atrophy and partial or complete shadowing posterior to the nevus.81 Compared with ophthalmoscopy, the technique detected the presence of subretinal fluid in twice as many eyes.81
In another study comparing EDI-OCT imaging of small choroidal melanoma with similar sized choroidal nevus, several characteristics were more commonly associated with melanoma: increased tumor thickness, subretinal fluid, subretinal lipofuscin deposition, RPE atrophy, intraretinal edema, loss of the external limiting membrane and the inner segment/outer segment junction, irregularity of the inner plexiform layer and the ganglion cell layer, photoreceptor loss, and “shaggy” swollen photoreceptors that appeared irregular and elongated.83
• Choroidal metastasis. Choroidal metastases are frequently a consequence of either lung or breast cancer.79 Lesions commonly appear within the posterior pole and macular regions and may be multifocal or even bilateral as is common in cases of breast cancer.79 Clinically, choroidal metastases appear amelanotic with indistinct margins.79
Researchers imaged 24 choroidal metastatic lesions with EDI-OCT and found that the majority demonstrated a plateau-shaped tumor with low internal reflectivity, overlying choriocapillaris thinning, shaggy photoreceptors, and subretinal fluid with high-reflective speckles.82
Additional associated features included RPE abnormality, photoreceptor abnormality, loss of the external limiting membrane or the inner segment/outer segment junction, and irregularity of the inner plexiform or ganglion cell layers.82
Five of the smaller tumors, which were undetected by ultrasonography, were identified and measured with EDI-OCT.82
Expanding Capabilities
Advancements in OCT technology, specifically the inclusion of enhanced depth imaging, allows for deeper evaluation of choroidal thickness and morphology. Emerging research suggests that choroidal dysfunction may play a role in the pathogenesis of many ocular diseases, even those initially thought to affect only the inner retina. The clinical uses of EDI-OCT include detection and monitoring of pathologic alterations in choroidal thickness, differentiation between diseases with similar clinical features, assessment of lamina cribrosa thickness in glaucoma patients, and precise measurement of choroidal tumor thickness.
Dr. Majcher is an assistant clinical professor at Rosenberg School of Optometry University of the Incarnate Word in San Antonio, Texas. Dr. Gurwood is a professor at Salus University in Elkins Park, Pa. Dr. Allen is a resident at the Minneapolis VA Medical Center in Minneapolis.
1. Lee J, Lee D, Lee J, et al. Correlation between subfoveal choroidal thickness and the severity or progression of nonexudative age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013 Nov 21;54(12):7812-8.2. Hirooka K, Fujiwara A, Shiragami C, et al. Relationship between progression of visual field damage and choroidal thickness in eyes with normal-tension glaucoma. Clin Experiment Ophthalmol. 2012 Aug;40(6):576-82.
3. Margolis R, Spaide R. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009 May;147(5):811-5.
4. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008 Oct;146(4):496-500.
5. Michalewska Z, Michalewski J, Nawrocki J. New OCT technologies take imaging deeper and wider: adding the possibility of imaging the choroid, retina, and vitreous. Retinal Physician. Available at: www.retinalphysician.com/articleviewer.aspx?articleID=108037. (accessed March 14, 2014).
6. Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol. 2013 Sep-Oct;58(5):387-429.
7. Carl Zeiss Meditec, Inc. (2011). Cirrus HD-OCT: How to read the Cirrus reports. Available at: http://www.meditec.zeiss.com/88256DE3007B916B/Order?OpenForm&RetinalImaging_EN.
8. Ding X, Li J, Zeng J, et al. Choroidal thickness in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2011 Dec 20;52(13):9555-60.
9. Manjunath V, Taha M, Fujimoto J, et al. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010 Sep;150(3):325-329.e1.
10. Ikuno Y, Kawaguchi K, Nouchi T, et al. Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci. 2010 Apr;51(4):2173-6.
11. Barteselli G, Chhablani J, El-Emam S, et al. Choroidal volume variations with age, axial length, and sex in healthy subjects: a three-dimensional analysis. Ophthalmology. 2012 Dec;119(12):2572-8.
12. Ho J, Branchini L, Regatieri C, et al. Analysis of normal peripapillary choroidal thickness via spectral domain optical coherence tomography. Ophthalmology. 2011 Oct;118(10):2001-7.
13. Wu L, Alpizar-Alvarez N. Choroidal imaging by spectral domain-optical coherence tomography. Taiwan Journal of Ophthalmology. 2013 March; 3 (1): 3-13.
14. Zhang L, Lee K, Niemeijer M, et al. Automated Segmentation of the Choroid from Clinical SD-OCT. Invest Ophthalmol Vis Sci.2012;53:7510e9.
15. Wei WB, Xu L, Jonas JB, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013 Jan;120(1):175-80.
16. Ayton LN, Guymer RH, Luu CD. Choroidal thickness profiles in retinitis pigmentosa. Clin Experiment Ophthalmol. 2013 May-Jun;41(4):396-403.
17. Tan CS, Ouyang Y, Ruiz H, et al. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012 Jan 25;53(1):261-6.
18. Usui S, Ikuno Y, Akiba M, et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 2012 Apr 24;53(4):2300-7.
19. Shinojima A, Iwasaki K, Aoki K, et al. Subfoveal choroidal thickness and foveal retinal thickness during head-down tilt. Aviat Space Environ Med. 2012;83:388-93.
20. Pournaras CJ, Logean E, Riva CE, et al. Regulation of subfoveal choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006 Apr;47(4):1581-6.
21. Grunwald JE, Metelitsina TI, Dupont JC, et al. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005 Mar;46(3):1033-8
22.Manjunath V, Goren J, Fujimoto JG, et al. Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmol. 2011 Oct;152(4):663-8.
23. Garg A, Oll M, Yzer S, et al. Reticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinning. Invest Ophthalmol Vis Sci. 2013 Oct 29;54(10):7075-81.
24. Kapoor K, Pulido J. Reticular pseudodrusen: a tale of two species? Eye (Lond). 2013 Jun;27(6):770-2.
25. Grunwald J, Hariprasad S, DuPont J, et al. Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1998 Feb;39(2):385-90.
26. Friedman E. A hemodynamic model of the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 1997 Nov;124(5):677-82.
27. Metelitsina T, Grunwald J, DuPont J, et al. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008 Jan;49(1):358-63.
28. Coscas F, Puche N, Coscas G, et al. Comparison of macular choroidal thickness in adult onset foveomacular vitelliform dystrophy and age-relatedmacular degeneration. Invest Ophthalmol Vis Sci. 2014 Jan 3;55(1):64-9.
29. Kim S, Oh, Kwon S, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011;31:1904–11.
30. Koizumi H, Yamagishi T, Yamazaki T, et al. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249:1123–1128.
31. Hu Z, Wu X, Coscas G, et al. Semiautomated segmentation of the choroid in spectral-domain optical coherence tomography volume scans. Invest Ophthalmol Vis Sci. 2013 Mar 7;54(3):1722-9.
32. Lee S, Fallah N, Forooghian F, et al. Comparative analysis of repeatability of manual and automated choroidal thickness measurements in nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013 Apr 23;54(4):2864-71.
33. Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond). 2010 Dec;24(12):1743-56.
34. Imamura Y, Fujiwara T, Margolis R, et al. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009 Nov-Dec;29(10):1469-73.
35. Kim SW, Oh J, Kwon SS, et al. Comparison of choroidal thickness among patients with healthy eyes, early age-related maculopathy, neovascular age-related macular degeneration, central serous chorioretinopathy, and polypoidal choroidal vasculopathy. Retina. 2011 Oct;31(9):1904-11.
36. Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011 Sep;31(8):1603-8.
37. Mehreen A, Jay D. Optical Coherence tomography – current and future applications. Curr Opin Opthalmol. 2013 May; 24(3): 213-221.
38. Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology. 2010 Sep;117(9):1792-9.
39. Maruko I, Iida T, Sugano Y, et al. One-year choroidal thickness results after photodynamic therapy for central serous chorioretinopathy. Retina. 2011 Oct;31(9):1921-7.
40. Chung SE, Kang SW, Lee JH, et al. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011 May;118(5):840-5.
41. Yang LH, Jonas JB, Wei WB. Optical coherence tomographic enhanced depth imaging of polypoidal choroidal vasculopathy. Retina. 2013 Sep;33(8):1584-9.
42. Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol. 2008 May;19(3):208-12.
43. Yannuzzi LA, Sorenson J, Spaide RF, et al. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990;10: 1–8.
44. Sasahara M, Tsujikawa A, Musashi K, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am J Ophthalmol 2006;142:601–7.
45. Kondo N, Honda S, Ishibashi K, et al. LOC387715/HTRA1 variants in polypoidal choroidal vasculopathy and age-related macular degeneration in a Japanese population. Am J Ophthalmol 2007;144:608–612.
46. Rishi P, Rishi E, Mathur G, et al. Ocular perfusion pressure and choroidal thickness in eyes with polypoidal choroidal vasculopathy, wet-age-related macular degeneration, and normals. Eye (Lond). 2013 Sep;27(9):1038-43.
47. Maruko I, Iida T, Sugano Y, et al. Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidalvasculopathy. Am J Ophthalmol. 2011 Apr;151(4):594-603.
48. Uyama M, Wada M, Nagai Y, et al. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 2002; 133: 639–48.
49. Burton AC. Relation of structure to function of the tissues of the walls of blood vessels. Physiol Rev 1954; 34: 619–42.
50. Koizumi H, Yamagishi T, Yamazaki T, et al. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2011 Aug;249(8):1123-8.
51. Nagase S, Miura M, Makita S, et al. High penetration optical coherence tomography with enhanced depth imaging of polypoidal choroidalvasculopathy. Ophthalmic Surg Lasers Imaging. 2012 Feb 9;43:e5-9.
52. Dhoot DS, Huo S, Yuan A, et al. Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br J Ophthalmol. 2013 Jan;97(1):66-9.
53. Falsini B, Anselmi GM, Marangoni D, et al. Subfoveal choroidal blood flow and central retinal function in retinitis pigmentosa. Invest Ophthalmol Vis Sci 2011;52:1064–9.
54. Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011.
55. Fryczkowski AW, Hodes BL, Walker J. Diabetic choroidal and iris vasculature scanning electron microscopy findings. Int Ophthalmol. 1989 Jul;13(4):269-79.
56. Fryczkowski AW, Sato SE, Hodes BL. Changes in the diabetic choroidal vasculature: scanning electron microscopy findings. Ann Ophthalmol. 1988 Aug;20(8):299-305.
57. Hidayat AA, Fine BS. Diabetic choroidopathy. Light and electron microscopic observations of seven cases. Ophthalmology. 1985 Apr;92(4):512-22.
58. Shiragami C, Shiraga F, Matsuo T, et al. Risk factors for diabetic choroidopathy in patients with diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2002 Jun;240(6):436-42.
59. Nagaoka T, Kitaya N, Sugawara R, et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol. 2004 Aug;88(8):1060-3.
60. Regatieri CV, Branchini L, Carmody J, et al. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012 Mar;32(3):563-8.
61. Esmaeelpour M, Považay B, Hermann B, et al. Mapping choroidal and retinal thickness variation in type 2 diabetes using three-dimensional 1060-nm optical coherence tomography. Invest Ophthalmol Vis Sci. 2011 Jul 15;52(8):5311-6.
62. Park SC, De Moraes CG, Teng CC, et al. Enhanced depth imaging optical coherence tomography of deep optic nerve complex structures in glaucoma. Ophthalmology. 2012 Jan;119(1):3-9.
63. Quigley HA, Hohman RM, Addicks EM, et al. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol 1983;95:673–91.
64. Bellezza AJ, Rintalan CJ, Thompson HW, et al. Deformation of the lamina cribrosa and anterior scleral canal wall in early experimental glaucoma. Invest Ophthalmol Vis Sci 2003;44: 623–37.
65. Park HY, Jeon SH, Park CK. Enhanced depth imaging detects lamina cribrosa thickness differences in normal tension glaucoma and primaryopen-angle glaucoma. Ophthalmology. 2012 Jan;119(1):10-20.
66. Lee EJ, Kim TW, Weinreb RN, et al. Visualization of the lamina cribrosa using enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2011 Jul;152(1):87-95.
67. Inoue R, Hangai M, Kotera Y, et al. Three-dimensional high-speed optical coherence tomography imaging of lamina cribrosa in glaucoma. Ophthalmology. 2009 Feb;116(2):214-22.
68. Tezel G, Trinkaus K, Wax MB. Alterations in the morphology of lamina cribrosa pores in glaucomatous eyes. Br J Ophthalmol 2004;88:251– 6.
69. Tatham AJ, Miki A, et al. Defects of the lamina cribrosa in eyes with localized retinal nerve fiber layer loss. Ophthalmology. 2014 Jan;121(1):110-8.
70. Maul EA, Friedman DS, Chang DS, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011 Aug;118(8):1571-9.
71. Ehrlich JR, Peterson J, Parlitsis G, et al. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res. 2011 Mar;92(3):189-94.
72. Usui S, Ikuno Y, Miki A, et al. Evaluation of the choroidal thickness using high penetration optical coherence tomography with long wavelength in highly myopic normal-tension glaucoma. Am J Ophthalmol. 2012 Jan;153(1):10-6.e1.
73. Park KH, Tomita G, Liou SY, et al. Correlation between peripapillary atrophy and optic nerve damage in normal-tension glaucoma. Ophthalmology. 1996 Nov;103(11):1899-906.
74. Quigley HA, Friedman DS, Congdon NG. Possible mechanisms of primary angle-closure and malignant glaucoma. J Glaucoma. 2003 Apr;12(2):167-80.
75. Huang W, Wang W, Gao X, et al. Choroidal Thickness in the Subtypes of Angle Closure: An EDI-OCT Study. Invest Ophthalmol Vis Sci. 2013 Dec 2;54(13):7849-53.
76. Yang M, Aung T, Husain R, et al. Choroidal expansion as a mechanism for acute primary angle closure: an investigation into the change ofbiometric parameters in the first 2 weeks. Br J Ophthalmol. 2005 Mar;89(3):288-90.
77. Zhou M, Wang W, Ding X, et al. Choroidal thickness in fellow eyes of patients with acute primary angle closure measured by enhance depth imaging spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013 Mar 19;54(3):1971-8.
78. Tailor TD, Gupta D, Dalley RW, et al. Orbital neoplasms in adults: clinical, radiologic, and pathologic review. Radiographics. 2013 Oct;33(6):1739-58.
79. Shields CL, Materin MA, Shields JA. Review of optical coherence tomography for intraocular tumors. Curr Opin Ophthalmol. 2005 Jun;16(3):141-54.
80. Shields CL, Shields JA. Clinical features of small choroidal melanoma. Curr Opin Ophthalmol. 2002 Jun;13(3):135-41.
81. Shah SU, Kaliki S, Shields CL, et al. Enhanced depth imaging optical coherence tomography of choroidal nevus in 104 cases. Ophthalmology. 2012 May;119(5):1066-72.
82. Demirci H, Cullen A, Sundstrom JM. Enhanced depth imaging optical coherence tomography of choroidal metastasis. Retina. 2013 Dec; 0 (0):1-6.
83. Shields CL, Kaliki S, et al. Enhanced depth imaging optical coherence tomography of small choroidal melanoma: comparison with choroidal nevus. Arch Ophthalmol. 2012 Jul;130(7):850-6.

