Based on physical signs and symptoms, optometrists can often make diagnoses in the primary optometric office. However, identifying the cause of a patient’s complaint won’t always be so easy. In some situations, imaging technology can help pinpoint a diagnosis and ultimately help the patient receive the best care.
The most common clinical indications for neuroimaging are: ptosis, proptosis, diplopia, ophthalmoplegia, nystagmus and optic disc abnormalities. Visual loss, visual field defects, anisocoria and other pupillary defects may also be an indication for neuroimaging, but only under particular circumstances.1
This article reviews various common imaging techniques and provides an overview of common neuro-ophthalmic disorders that may require neuroimaging.
Techniques
Compared with modern options, such as computed tomography (CT) and magnetic resonance imaging (MRI), x-ray is somewhat antiquated. However, it still can be beneficial to eye care professionals in instances when you want a patient to get an MRI but they have a history of metal in the eye; a plain film x-ray can detect any remaining metallic foreign body in the orbit, which would be a contraindication to MRI testing. An x-ray may also be used following facial trauma to evaluate the orbit for a fracture.
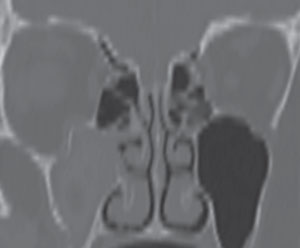 | |
| Fig. 1. The inferior aspect of the right orbit has been fractured and the maxillary sinus is opacified in this computed tomography scan, which uses both x-rays and sensors to gather data and make soft tissue visible. In these scans, air-filled pockets are black. |
Computed Tomography
A CT scan uses both x-rays and sensors. A computer processes the data gathered by the sensors to make organs and other soft tissues viewable, which provides for greater analysis of the head and neck region. When viewing a CT scan with or without contrast, the bones are visible as white and the air-filled spaces, similar to an x-ray, appear black. Soft tissue appears in gray tones. The differences in the gray tones are a result of the soft tissue’s various densities. As an example, the extraocular muscles are a lighter gray than the brain.
CT is both cost- and time-efficient in providing vital information with respect to the brain, orbit and bone. It is the imaging modality of choice for evaluating acute hemorrhage, calcification and bony abnormalities. CT scanning is painless, noninvasive, accurate and less sensitive to patient movement than MRI. Unlike MRI, CT can also be performed on patients with implanted medical devices of any kind. Disadvantages of CT include ionizing radiation, beam-hardening artifacts and iodinated contrast-induced allergy. Because of the ionizing radiation, CT imaging is not indicated in children and pregnant women. CT has a weight limit of approximately 450lbs. Although CT depicts soft tissue to some degree, an MRI would be the better study to evaluate the brain and orbital soft tissues.
You can order a CT scan with and without iodine-based contrast. CT contrast agents are used to highlight specific areas within organs, blood vessels and tissues. Following iodine injection into the bloodstream, the CT’s x-ray beam generally weakens as it passes through blood vessels. Organs that have taken up the contrast are bright on the CT images. When the test is finished, the kidneys and liver eliminate the contrast from the body. Because of this, use care with patients who have kidney dysfunction, as well as individuals who may have iodine sensitivities.
In eye care, a CT scan is often used to image the bony orbit and view the anatomic position of the extraocular muscles (EOM). In instances when a patient presents with orbital trauma, a non-contrast CT of the orbit can be advantageous. Clinical signs that help to identify patients who require imaging include: resistance to forced duction, diplopia, afferent pupillary defect, bony displacement of the orbital globe, orbital crepitus, enophthalmos and subconjunctival hemorrhage, which can indicate a retrobulbar hemorrhage. One clinical caveat is when a patient suffers orbital trauma and presents with significant ecchymosis but with full range of motion and no evidence of globe displacement. In these cases, it is helpful to determine the status of the maxillary division (V2) of the 5th cranial nerve by gently touching the fibrous end of a cotton tip applicator along the V2 distribution. Denervation within V2 will confirm the need for emergent non-contrast orbital CT to evaluate for a break in the orbital floor and to discount a hemorrhage within the sinus cavities. In patients who present with ophthalmoplegia, specifically an inability to look upward, it is prudent to suspect that there may be inferior rectus entrapment from an inferior orbital floor fracture (Figure 1).
CT in Practice
The following clinical cases will highlight the importance of CT imaging:
Scenario One. A 75-year-old male presents with a chief complaint of acute peripheral vision loss. The patient recently underwent a comprehensive eye exam, and the findings at that time were unremarkable. During the current exam, pupillary responses are sluggish, and confrontation fields reveal a right homonymous defect.
You ask the patient how long he has noticed the visual field defect and whether it started acutely and note that the patient appears confused and not overly attentive. In addition, he has a mild facial paralysis. You now suspect the patient may have had a stroke, and at the completion of the exam the patient indicates an excruciating headache. With the suspicion of a cerebrovascular accident (CVA) and the new headache symptom, a STAT referral to the emergency room is warranted. At the emergency room, a CT scan will likely be done to rule out a hemorrhagic stroke due to the neurologic changes and the acute headache.2,3
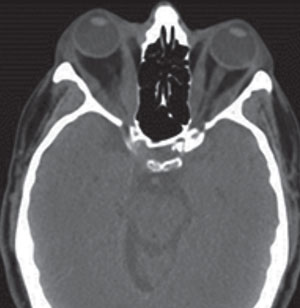 | |
| Fig. 2. The thickening of the extraocular muscle bellies ultimately causes the eyes to become prophetic. Photo: Denise Goodwin, OD. |
Scenario Two. A 57-year-old female presents with a chief complaint that her eyes are “bulging out.” Her systemic history is notable for hypertension and, she says, she was told she has “thyroid problems.” During the clinical examination, you grossly observe bilateral exophthalmos and restricted extraocular motilities. The patient also reports concomitant diplopia. Though the patient is thin, you notice that her anterior neck appears enlarged and suspicious for goiter. Best-corrected acuities are 20/20 in both eyes. Along with the exophthalmos, there is a moderate amount of punctate keratitis. Dilated fundus evaluation is unremarkable. With these findings you suspect an orbital process most likely related to Graves’ disease and thyroid eye disease. You refer the patient to an endocrinologist for further evaluation. Laboratory testing indicates a low thyroid stimulating hormone (TSH) and an elevated free thyroxine (T4) level. In this instance, a CT will allow for localization of the small bones of the orbit and can help the clinician assess the orbit and its structures for possible fracture or hemorrhage.4,5 In this case, CT will also help the clinician evaluate for enlarged extraocular muscle bellies (Figure 2). The orbital coronal CT image will be helpful in evaluating the proximity of the extraocular muscles to the optic nerve at the orbital apex; compressive optic neuropathy needs to be considered in these patients.
Magnetic Resonance Imaging
MRI also provides valuable imaging of the orbital soft tissues and the brain.6,7 Unlike a traditional x-ray or CT, MRI analyzes biological tissues using magnetic fields and radio waves, providing a detailed image of the soft tissue. Note: MRI is contraindicated in patients with potentially loose magnetic metallic items such as cochlear implants, aneurysm clips, pacemakers, defibrillators, spinal stimulators and other electronic medical devices. Some implantable devices may be MRI conditional, meaning that an MRI may be allowed under certain conditions, such as specific magnet strengths. You can contact the manufacturer of the device to get the specific conditions under which an MRI can be safely obtained and share this information with the radiology facility. When in doubt, consult with the radiologist and other health professionals involved.
The advantage of MRI is enhanced definition between different soft tissues, making it the procedure of choice for imaging the soft tissue of the brain and orbit. Unlike CT, MRI is not contraindicated in pregnant patients or children. MRI is advantageous in diagnosing early-onset cerebrovascular accident, multiple sclerosis (MS), tumors (e.g., meningioma), inflammation and infection.
Viewing Angles
|
As many patients feel claustrophobic in a traditional MRI, modern technology now allows for an open MRI, and even a variant that allows patients to be seated. Though these options are available, there is widespread understanding that the tubular version provides better images.8 Of course, if the patient is unable to lie flat, or cannot undergo traditional MRI testing, these options are better than not being able to get any images.8
MRI uses three primary techniques: proton density, T1- and T2-weighted sequences.
Proton density scans provide an image depicting the density of protons in tissue. A T1-weighted scan represents the time it takes tissue to recover from a radiofrequency pulse. With T1-weighted images, fluid is extremely dark, water-based tissue is gray and lipid-based tissues are bright. A T2-weighted scan represents the time the signal lasts after giving a radiofrequency pulse. Clinically, it results in water-based tissues being bright and lipid-based tissues appearing darker. A T2-weighted scan uses a gradient echo (GRE) sequence, which is most often used in suspected cases of intracranial microhemorrhage. These images provide good contrast between gray and white matter in the brain, iron-laden tissues and venous vessels.
Inversion recovery, fluid attenuated inversion recovery (FLAIR) and standard short T1-inversion recovery (STIR) are techniques used to suppress certain anatomic tissue. FLAIR sequences produce heavily T2-weighted images with suppression of cerebrospinal fluid, allowing for more sensitive resolution in suspected cases of demyelination or lesions anatomically close to the ventricles. Clinically speaking, this is the imaging sequence of choice when ordering an MRI for patients with optic neuritis. STIR sequence can be used with T1- or T2-weighted images to obtain fat signal suppression. Other pulse sequences of MRI include magnetization transfer contrast, diffusion-weighted, echo planar, perfusion and functional.9
Gadolinium contrast is often administered intravenously prior to an MRI to increase the visibility of intracranial infection, inflammation, early ischemia and meningeal lesions. It also helps to distinguish active demyelinating plaques from quiescent ones. Prior to ordering an MRI with contrast, a patient should have labs performed to check for adequate kidney function, including serum creatinine and blood urea nitrogen (BUN).10 Individuals with a history of gadolinium allergies, those who have kidney impairment and those who take medications excreted through the kidneys are at risk when using contrast. Usually the kidney functions need to be collected within four to six weeks of the use of contrast; the exact time limitations are facility dependent. In some instances, diabetic patients who take metformin may be advised to forego taking their medications for two days prior to an MRI with contrast.10 For orbital studies, MRI with and without contrast should be considered to rule out mass lesions or other potential findings.
MRI in Practice
An MRI may be beneficial for eye care practitioners in these scenarios:
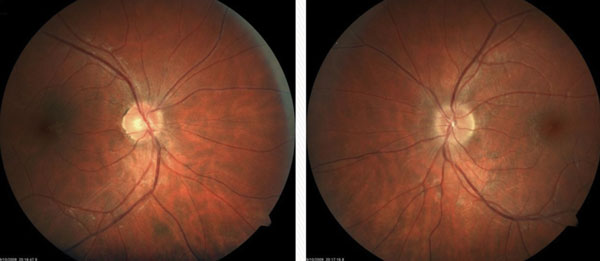 |
| Fig. 3. A patient’s fundus images showed no abnormalities of the right eye. However, in the left eye the image shows a crowded nerve with blurred margins. |
Scenario One. A 28-year-old female presents to the office with vague visual symptoms. She indicates that she has blurry vision only intermittently. The periods of poor vision have occurred several times throughout the last five years, but since vision returns to normal after a couple weeks, she never felt the need for an eye exam. Since her vision has been reduced for the past two weeks without improvement, she felt it was necessary to have an examination. Preliminary testing revealed normal extraocular motilities and confrontation visual fields. Pupil evaluation elicited 1+ RAPD in the left eye. Red cap testing revealed desaturation of the left eye. With a minimal refractive error, best-corrected visual acuities were 20/20 OD and 20/25- OS. Dilated fundus examination revealed no abnormalities of the right eye. The left eye had a crowded nerve with blurred margins (Figure 3). Because the macular region was unremarkable, a diagnosis of optic neuritis was suspected and a referral to a neurologist, or neuro-ophthalmologist, is necessary since optic neuritis can be associated with demyelination and MS.11 If findings are atypical for a diagnosis of demyelinating optic neuritis, laboratory testing can rule out an infectious etiology such as Lyme disease, syphilis or tuberculosis.12 An MRI is warranted in cases of optic neuritis to confirm the optic nerve inflammation and to look for the classic white matter lesions perpendicular to the lateral ventricles, consistent with MS (Figure 4).13 The lesions associated with MS are most easily seen with the FLAIR MRI sequence. With T1-weighted imaging, active lesions may also enhance with contrast.14 It should be noted that the patient may be relatively asymptomatic and still have lesions; MRI findings may not make the diagnosis of MS, particularly if patients are asymptomatic.15 The imaging and proper diagnosis leads to a prompt treatment, which can speed visual recovery and maintain quality of life as best as possible, since it decreases as the disease progresses.16
Scenario Two. A 55-year-old male patient presents with complaints of increasing difficulty reading the newspaper, headaches and decreased vision in the right eye. Systemic health is unremarkable. Pupillary testing reveals a 1+ RAPD of the right eye and color vision deficiency in the right eye. Confrontation visual fields reveal an inferior temporal defect in the right eye. The visual field is full for the left eye. Best-corrected acuities are 20/40 OD and 20/20 OS. Anterior segment examination is unremarkable. Dilated fundus evaluation is unremarkable for the left eye, but the right optic nerve has blurred disc margins.
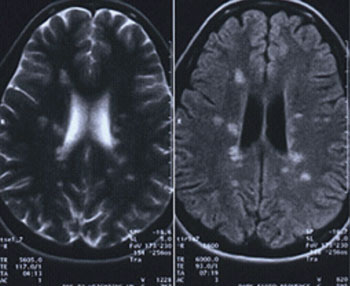 | |
| Fig. 4. The patient’s MRI reveals classic white matter lesions perpendicular to the lateral ventricles, consistent with MS. These lesions are most easily seen using th FLAIR MRI sequence. T1-weighted imaging may also help identify any active lesions. |
A clinician must consider a neurologic cause to these signs and symptoms, and neuroimaging is warranted. In this case, an MRI of the brain and orbits with and without contrast reveals an optic nerve glioma.17
Scenario Three. A 54-year-old male presents indicating that his progressive bifocals are inadequate for his near tasks. Preliminary testing revealed normal pupillary responses and extraocular motilities, but there was a restricted temporal visual field in each eye. Best-corrected vision was 20/25 in each eye at distance and near. Dilated fundus examination was unremarkable for all structures except the optic discs. The optic discs showed temporal pallor bilaterally. A Humphrey visual field 24-2 was performed, revealing a superior temporal defect in each eye. Based on the clinical findings, the optometrist would suspect a lesion in the suprasellar cistern, most commonly a pituitary adenoma, and would refer for the appropriate imaging to confirm the diagnosis. Prompt confirmation of the pituitary adenoma can streamline the process to neuro-surgical intervention, which is important because continued compression of the chiasm may lead to irreversible optic nerve damage. Patients with severe headache and sudden bitemporal vision loss need to be imaged immediately to rule out pituitary apoplexy. Pituitary apoplexy and adenoma can both cause hormonal changes, as the pituitary gland is responsible for many endocrine functions.18 Because of the risk for adrenal failure and death from untreated pituitary apoplexy, patients suspected of having this need to be sent to the ER rather than having outpatient imaging.19
Neurovascular Imaging
The ability to visualize the neurovascular anatomy involves expressing the blood vessels and suppressing the surrounding structures. In digital subtraction angiography (DSA) and computerized tomography angiography (CTA), iodinated intravascular contrast is injected to create a detailed analysis of the vascular system. For patients with iodine sensitivity or kidney dysfunction, magnetic resonance angiography (MRA) is a safe alternative.
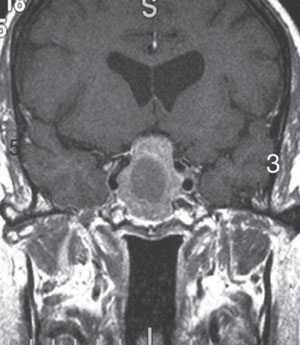 | |
| Fig. 5. T1-weighted MRI with gadolinium can help confirm a diagnosis of a pituitary adenoma. Photo: Heather Shelsta, MD. |
CTA. Compared with DSA, both CTA and MRA are relatively noninvasive procedures for studying the vascular system. The advantages of CTA over MRA include: cost, accessibility, less time consuming, applicable for patients with implantable devices and more conducive to those with claustrophobia. Clinically, CTA can detect aneurysms as small as 1.7mm and is superior for imaging aneurysms of the head and neck, characterizing thrombi, and detecting vasospasm, arterial stenosis and carotid-cavernous fistulas. The drawbacks of CT angiography include difficult detection and delineation of cavernous sinus and posterior inferior cerebellar artery aneurysms, feeding vessels for dural carotid cavernous fistulas and risks involving radiation exposure and contrast agents.20,21
MRA. With MRA, a low dose of gadolinium contrast is injected into a vein, and images are acquired during the first pass of the agent through the arteries. MRA can help clinicians evaluate the extracranial circulation (i.e., carotid artery stenosis, plaques and dissections in the evaluation of transient visual loss) and intracranial circulation (i.e., aneurysms, arteriovenous malformations, occlusive disease and carotid artery fistulas). The limitations of MRA include the possibility of false-positive results in tightly wound vessel loops, as well as a tendency to exaggerate vessel stenosis.22
DSA. This provides images of blood vessels by inserting a tube into a large artery and threading it through the circulatory system, and then injecting the tube with contrast dye. A series of radiographs is taken as the contrast spreads throughout the arterial system. A second series of radiographs is taken as the dye exits via the venous system. DSA allows clinicians to perform endovascular treatment immediately based on the findings. However, major complications in DSA do exist, including: CVA, allergic reaction to the anesthetic or contrast medium, damage to one of the access veins or thrombosis and embolism formation. Minor complications include bleeding or bruising at the site of the dye injection.
The most common uses of DSA in neuro-ophthalmic disorders is to detect and localize small intracranial aneurysms in the presence or absence of sub-arachnoid blood. It is widely used in interventional neuroradiology, as it allows for detailed anatomy of the vasculature in order to introduce flexible microcatheters, balloons, coils, chemical agents and other devices.23 Common indications are for evaluating and treating arteriovenous malformations and carotid cavernous and dural fistulas with intracranial coils.23
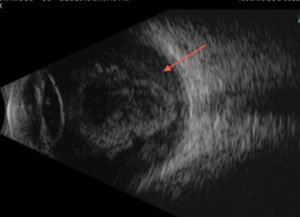 | |
| Fig. 6. The retina, which is derived from the neuroectoderm, can be imaged using ultrasound, such as this B-scan of a retinal detachment. |
In general, MRI is the imaging modality of choice for most neuro-ophthalmological disorders, while CT is advantageous for viewing the skull, bony orbit, EOM entrapment and blood following head and facial trauma. Because MRI has many different imaging sequences, it allows for detailed assessment of soft tissue. When ordering a neuroimaging study, with the exception of T1- and T2-weighted images, it is important to provide the radiologist with specific sequences and state what you are looking for to tailor the study to the patient’s needs. In addition, the nature of the suspected pathology is also important in deciding the protocol and if contrast is required. If there is any doubt as to which imaging study is recommended, consult with the neuroradiologist. Direct communication with the interpreting physician will decrease the chance of omitting important clinical information and will allow them to focus their attention on the area(s) of concern.
Dr. Suhr practices at the New Port Richey Department of Veterans Affairs Outpatient Clinic, in New Port Richey, Fla.
Dr. DelGiodice is in practice at Associated Eye Physicians in Clifton, NJ.
1. Johnson M, Policeni B, Lee A, Smoker W. Neuroimaging in Ophthalmology. New York, NY: Oxford University Press; 2011.2. Walecki J, Mruk B, Nawrocka-Laskus E, et al. Neuroimaging of cerebral venous thrombosis (CVT)-old dilemma and the new diagnostic methods. Pol J Radio. 2015 July;80:368-73.
3. Brouwers H, Battey T, Musial H, et al. Rate of contrast extravasation on computed tomographic angiography predicts hematoma expansion and mortality in primary intracerebral hemorrhage. Stroke. 2015 Sep;46(9):2498-503.
4. Rabinowitz M, Carrasco J. Update on advanced imaging options for thyroid-associated orbitopathy. Saudi J Ophthalmol. 2012 Oct;26(4):385-92.
5. Kirsch E, von Arx G, Hammer B. Imaging in Graves’ orbitopathy. Orbit. 2009;28(4):219-25.
6. Verma R, Gupta M, Mehta V. Thyroid associated orbitopathy. BMJ Case Rep. 2013 June. [Epub].
7. Daubner D, Spieth S, Engellandt K, von Kummer R. Diagnosis and differential diagnosis of Graves’ orbitopathy in MRI. Radiologe. 2012;52(6):550-9.
8. Hailey D. Open magnetic resonance imaging (MRI) scanners. Issues Emerg Health Technol. 2006 Nov;(92):1-4.
9. van Everdingen KJ. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke. 1998 Sept;29(9):1783-90.
10. American College of Radiology (ACR) Committee on Drugs and Contrast Media. ACR Manual on contrast Media. 2015;10. www.acr.org/~/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf.
11. van der Walt A, Kolbe S, Mitchell P. Parallel changes in structural and functional measures of optic nerve myelination after optic neuritis. PLoS One. 2015 May;10(5):1-11.
12. Kemp PS, Winges, KM, Wall M. Optic neuritis. EyeRounds.org. Posted Sept. 30, 2012. www.EyeRounds.org/cases/159-optic-neuritis.htm.
13. Sasaki T, Nakayama T, Kitamura M, et al. Case of 55-year-old female with primary central nervous system lymphoma, presenting with brain and eye lesions and long-term relapsing/remitting course. Rinsho Shinkeigaku. 2015 Aug 21;55(8):567-72.
14. Patrella JR, Grossman RI, McGowan JC, et al. Multiple sclerosis lesions: relationship between MR enhancement pattern and magnetization transfer effect. AJNR AM J Neuroradiol. 1996 June;17(6):1041-9.
15. Barkhof F, Simon JH, Fazekas F, et al. MRI monitoring of immunomodulation in relapse-onset multiple sclerosis trials. Nat Rev Neurol. 2011;8(1):13-21.
16. Tauhid S, Chu R, Sasane R, et al. Brain MRI lesions and atrophy are associated with employment status in patients with multiple sclerosis. J Neurol. 2015 Jul 24. [Epub ahead of print].
17. Matoob S, Fan JC, Danesh-Meyer HV. Multifocal malignant optic glioma of adulthood presenting as acute anterior optic neuropathy. J Clin Neurosci. 2011 Jul;18(7):974-7.
18. Weed MC, Longmuir RA, Thurtell MJ. Pituitary adenoma causing compression of the optic chiasm: 49-year-old white female with painless progressive vision loss. www.EyeRounds.org. September 9, 2013.
19. Xu K, Yuan Y, Zhou J, Yu J. Pituitary adenoma apoplexy caused by rupture of an anterior communicating artery aneurysm: Case report and literature review. World J Surg Oncol. 2015 Jul 30;13(1):228.
20. Chow LC, Rubin GD. CT angiography of the arterial system. Radiol Clin North Am. 2002 Jul;40(4):729-49.
21. Goddard AJ, Tan G, Becker J. Computed tomography angiography for the detection and characterization of intra-cranial aneurysms: current status. Clin Radiol. 2005 Dec;60(12):1221-36.
22. Sands MJ, Levitin A. Basics of magnetic resonance imaging. Semin Vasc Surg. 2004 Jun;17(2):66-82.
23. Wintermark M, Meuli R, Browaeys P, et al. Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment. Neurology. 2007 Feb 27;68(9):694-7.